Solved Calculations Part 1 Determine The Ksp For Each Chegg

Solved Show All Calculations 1 Determine The Ksp Chegg Use the ph value to determine the hydroxide ion concentration at equilibrium. here’s the best way to solve it. 4. calculate the [ag ] in mol l (m) needed to begin precipitation of each of these anions from solutions containing a concentration of 500 p.p.m. for each anion.
Solved Perform Calculations Using Ksp Question For The Chegg I note that the molar relationship is a 1:1 molar ratio, meaning that for every one mole of agcl that dissolves, one mole of ag is produced. that leads me to this:. Using the two values of Δgo at the two temperatures, solve for Δho and Δso. (note: could you alternatively determine these two parameters using the van't hoff relationship?). Find the accepted value of the ksp for calcium hydroxide and compare it with your value. discuss the discrepancy and suggest possible sources of experimental error. Example #1: a solid sample of ca (oh) 2 is shaken with 0.0100 m cacl 2. once equilibrated, some solid ca (oh) 2 remains undissolved. the solution is filtered and a 25.00 ml sample requires 22.50 ml of 0.0250 m hcl to neutralize it. calculate the value for k sp of ca (oh) 2 from this data.
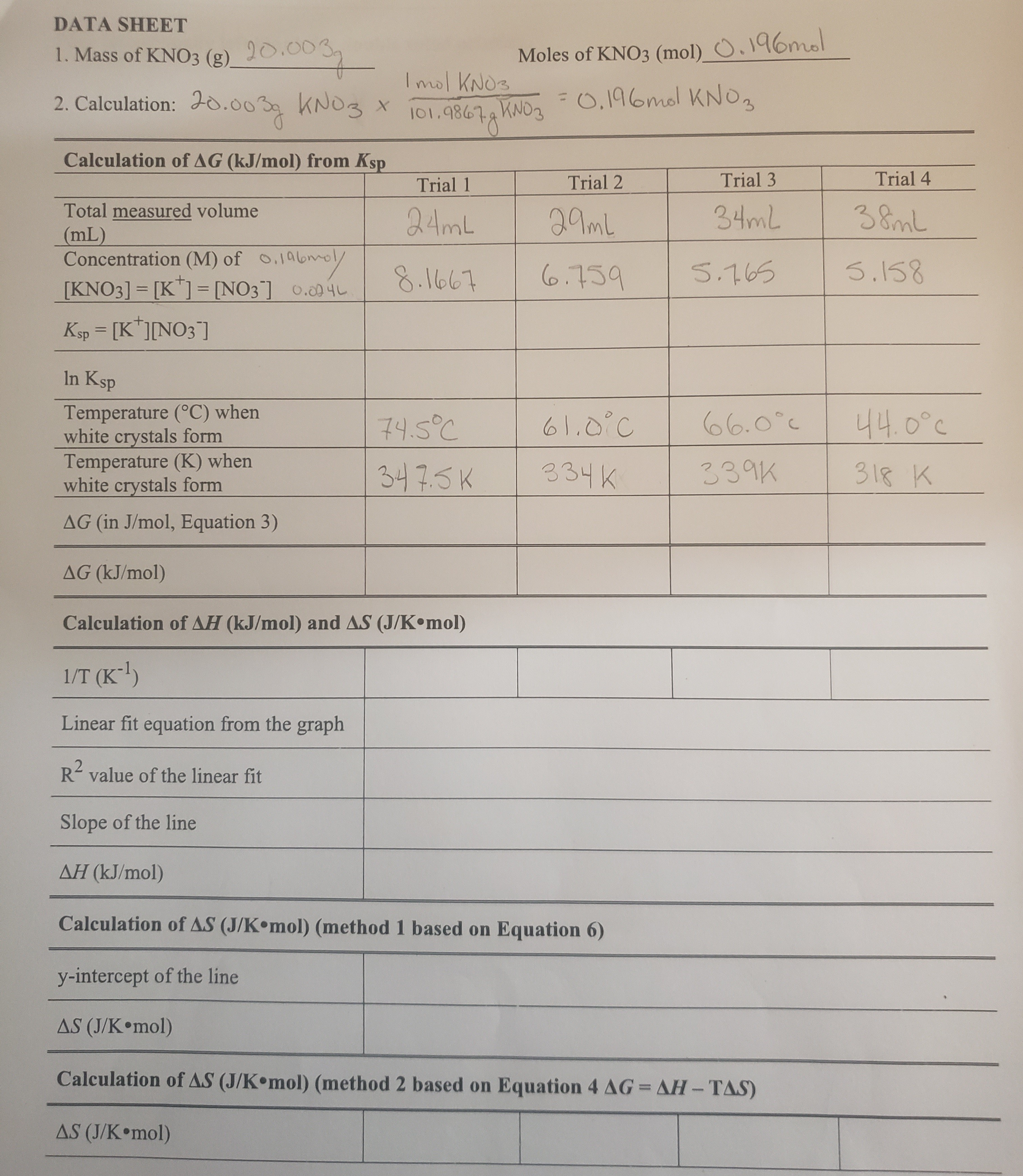
Solved How To Calculate Ksp Based On My Calculations Are Chegg Find the accepted value of the ksp for calcium hydroxide and compare it with your value. discuss the discrepancy and suggest possible sources of experimental error. Example #1: a solid sample of ca (oh) 2 is shaken with 0.0100 m cacl 2. once equilibrated, some solid ca (oh) 2 remains undissolved. the solution is filtered and a 25.00 ml sample requires 22.50 ml of 0.0250 m hcl to neutralize it. calculate the value for k sp of ca (oh) 2 from this data. Solubility data can be used to calculate the k s p for a given compound. the following steps need to be taken. convert from solubility to molar solubility. apply the k s p equation. sample problem: calculating k s p from solubility. Learn to calculate ksp, solubility, and compare ksp values. chemistry worksheet for high school early college students. Question: part 1: determine the ksp and % error for ca (oh)2, cu (oh)2, ba (oh)2, and mg (oh)2 solutions. follow the exact steps outlined for the ca (oh)2 solution. Example #1: calculate the solubility product of agi at 25.0 °c, given the following data:.

Solved Part B Average Ksp Standard Deviation Chegg Solubility data can be used to calculate the k s p for a given compound. the following steps need to be taken. convert from solubility to molar solubility. apply the k s p equation. sample problem: calculating k s p from solubility. Learn to calculate ksp, solubility, and compare ksp values. chemistry worksheet for high school early college students. Question: part 1: determine the ksp and % error for ca (oh)2, cu (oh)2, ba (oh)2, and mg (oh)2 solutions. follow the exact steps outlined for the ca (oh)2 solution. Example #1: calculate the solubility product of agi at 25.0 °c, given the following data:.
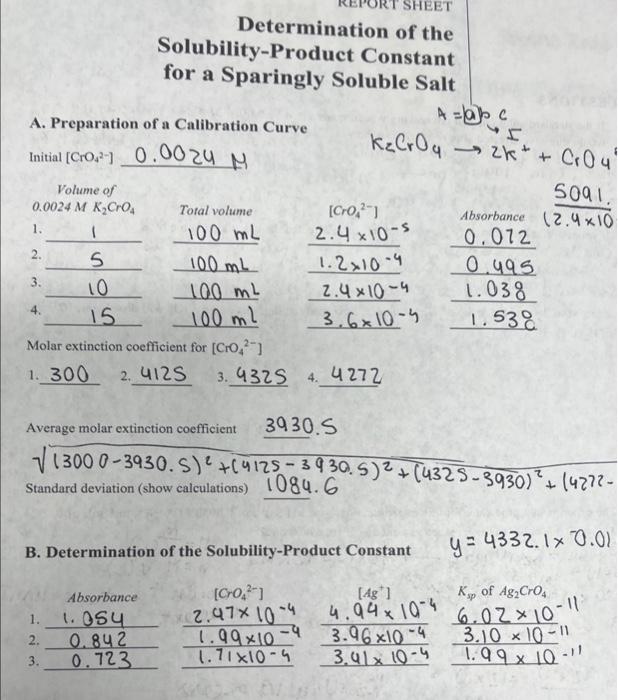
Solved Give Me The Average Ksp Show Calculations And The Chegg Question: part 1: determine the ksp and % error for ca (oh)2, cu (oh)2, ba (oh)2, and mg (oh)2 solutions. follow the exact steps outlined for the ca (oh)2 solution. Example #1: calculate the solubility product of agi at 25.0 °c, given the following data:.
Comments are closed.