Solved Chem 182 Experiment 8 Determining The Ksp Of Calcium Chegg

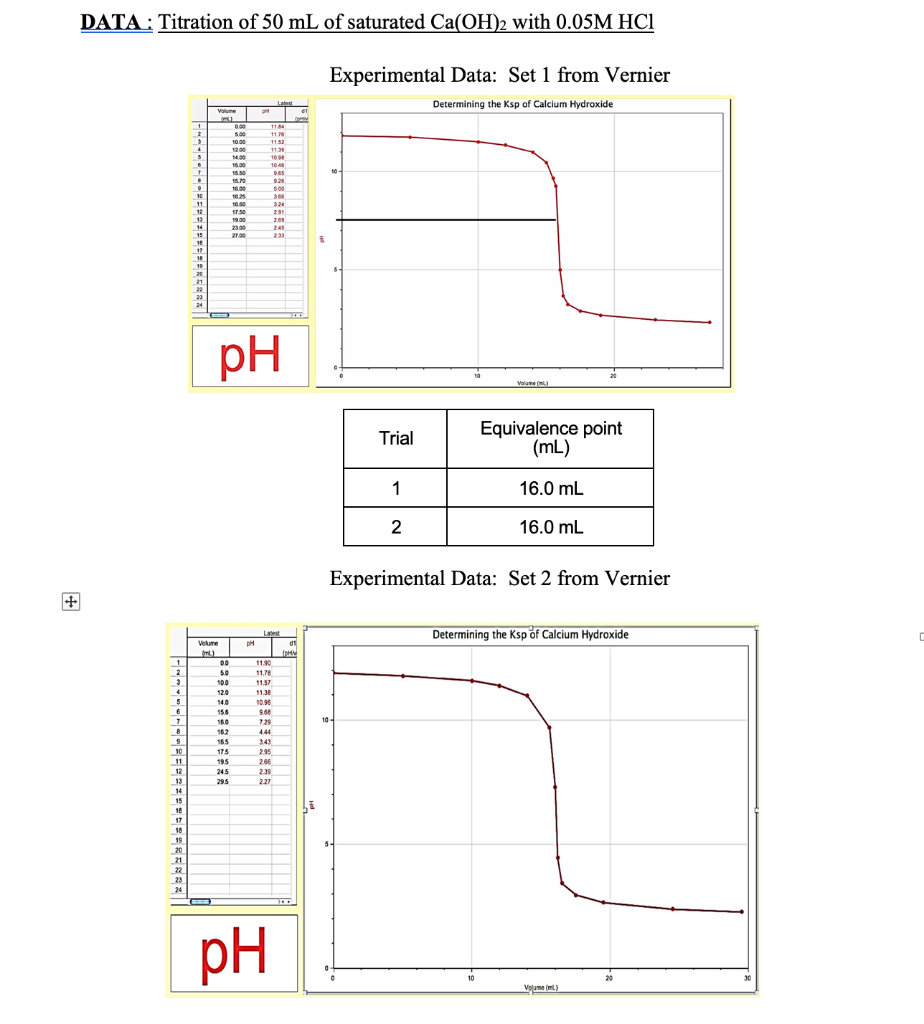
Solved Chem 182 Experiment 8 Determining The Ksp Of Calcium Chegg For this experiment, it is important to know that the solubility of the aqueous dissolution of calcium hydroxide decreases as the temperature increases. to determine the ksp and equivalence points for part a and part b of this experiment, we had to do a titration procedure. Chem 182: experiment 8 determining the ksp of calcium hydroxide calcium hydroxide is an ionic solid that is sparingly soluble in water solution of ca (oh)2 is represented in equation form lution of ca (on s nronic solid that is sparingly soluble in.

Solved Pre Lab Exercise Name Riment 8 Determining The Ksp Chegg Your primary objective in this experiment is to test a saturated solution of calcium hydroxide and use your observations and measurements to calculate the ksp of the compound. Your primary objective in this experiment is to test a saturated solution of calcium hydroxide and use your observations and measurements to calculate the k sp of the compound. you will do this by titrating the prepared ca (oh) 2 solution with a standard hydrochloric acid solution. The experiment aims to determine the solubility product constant (ksp) of calcium hydroxide (ca (oh)2) in water and investigate the effect of temperature on its solubility. Write the ksp expression for ca (oh)2 and al (oh)3. 3. calculate ksp of ca (oh)2 if its solubility is 0.458 g l. 4. what factors can influence the value of ksp. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on.

Solved Chi 169 7 Determining The Ksp Of Calcium Hydroxide Chegg The experiment aims to determine the solubility product constant (ksp) of calcium hydroxide (ca (oh)2) in water and investigate the effect of temperature on its solubility. Write the ksp expression for ca (oh)2 and al (oh)3. 3. calculate ksp of ca (oh)2 if its solubility is 0.458 g l. 4. what factors can influence the value of ksp. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. In this experiment, the kspfor calcium hydroxide, ca (oh)2 will be determined through the technique of titration when dissolved in water. The purpose of this lab was to determine ksp for calci um hydroxide. the following is the.

Solved Chem 182 Experiment 8 Determining The Ksp Of Calcium Chegg In this experiment, the kspfor calcium hydroxide, ca (oh)2 will be determined through the technique of titration when dissolved in water. The purpose of this lab was to determine ksp for calci um hydroxide. the following is the.

Solved Alt Chem 125 Name Determining The Ksp Of Calcium Chegg
Solved Determining The Ksp Of Calcium Hydroxide Introduction Chegg
Comments are closed.