Solved Consider The Following Equilibrium 2 Nocl G Chegg
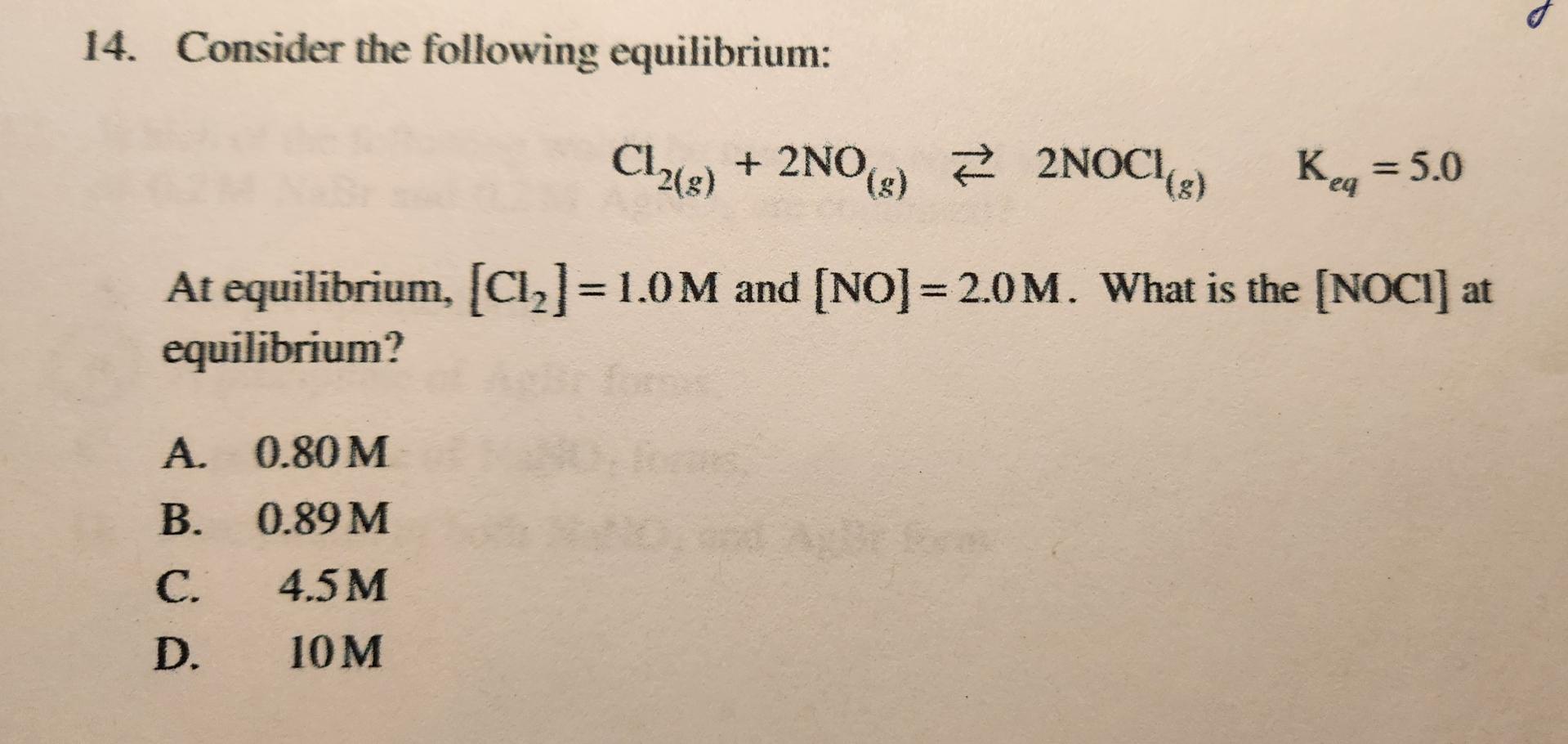
Solved Consider The Following Chegg Science chemistry chemistry questions and answers consider the following equilibrium: 2 nocl (g) 2 no (g) cl2 (g) with kc = 1.6. Calculate the equilibrium concentration of cl 2 (g). upload your school material for a more relevant answer. the equilibrium concentration of cl2 (g) can be calculated using the given initial amounts of nocl and cl2, the stoichiometry of the balanced equation, and the equilibrium constant (k).

Solved Consider The Following Equilibrium Chegg Answer to solve this problem, we need to understand the concept of equilibrium constants and how they relate to the concentrations (or in this case, partial pressures) of reactants. Since $$\delta g^ {0}$$Δg0 is positive (41 kj), the equilibrium favors the reactants. a q value of 0 indicates that the reaction will proceed to the right (towards products), thus decreasing the pressure of nocl. We are given with the gibbs free energy of the equation as well as the temperature, so we can easily solve for k. we know the equation of k: k= products reactants and since we are given with the partial pressure of cl gas ans nocl, we can solve partial pressure of no. is this answer helpful?. Consider the following equilibrium reaction: 2 nocl (g) ⇌ cl2 (g) 2 no (g) if the volume is increased, in which direction will the reaction shift to reestablish equilibrium?.

Solved Consider The Following Equilibrium Chegg We are given with the gibbs free energy of the equation as well as the temperature, so we can easily solve for k. we know the equation of k: k= products reactants and since we are given with the partial pressure of cl gas ans nocl, we can solve partial pressure of no. is this answer helpful?. Consider the following equilibrium reaction: 2 nocl (g) ⇌ cl2 (g) 2 no (g) if the volume is increased, in which direction will the reaction shift to reestablish equilibrium?. Question: consider the following equilibrium reaction: 2 nocl (g) ⇌ cl2 (g) 2 no (g) if the volume is increased, in which direction will the reaction shift to reestablish equilibrium?. The equilibrium 2no (g) cl2 (g)⇌2nocl (g) is established at 500 k. an equilibrium mixture of the three gases has partial pressures of 9.80×10−2 atm , 0.175 atm , and 0.27 atm for no, cl2, and nocl, respectively. Basically, you don't do the quadratic equation and ignored the " x" or " x" when solving for x, but then put it back to solve for the equilibrium concentrations. Consider the following equilibrium: 2nocl (g) < > 2no (g) cl 2 (g) k = 1.6 x 10 5 1.00 mole of pure nocl and 0.958 mole of pure cl 2 are placed in a 1.00 l container. calculate the equilibrium concentration of no (g). provide all work and equations used for correct answer.

Solved Consider The Equilibrium Cl 2 G 2 No G 2 Chegg Question: consider the following equilibrium reaction: 2 nocl (g) ⇌ cl2 (g) 2 no (g) if the volume is increased, in which direction will the reaction shift to reestablish equilibrium?. The equilibrium 2no (g) cl2 (g)⇌2nocl (g) is established at 500 k. an equilibrium mixture of the three gases has partial pressures of 9.80×10−2 atm , 0.175 atm , and 0.27 atm for no, cl2, and nocl, respectively. Basically, you don't do the quadratic equation and ignored the " x" or " x" when solving for x, but then put it back to solve for the equilibrium concentrations. Consider the following equilibrium: 2nocl (g) < > 2no (g) cl 2 (g) k = 1.6 x 10 5 1.00 mole of pure nocl and 0.958 mole of pure cl 2 are placed in a 1.00 l container. calculate the equilibrium concentration of no (g). provide all work and equations used for correct answer.

Solved Consider The Following Reaction At Equilibrium 2 No Chegg Basically, you don't do the quadratic equation and ignored the " x" or " x" when solving for x, but then put it back to solve for the equilibrium concentrations. Consider the following equilibrium: 2nocl (g) < > 2no (g) cl 2 (g) k = 1.6 x 10 5 1.00 mole of pure nocl and 0.958 mole of pure cl 2 are placed in a 1.00 l container. calculate the equilibrium concentration of no (g). provide all work and equations used for correct answer.
Comments are closed.