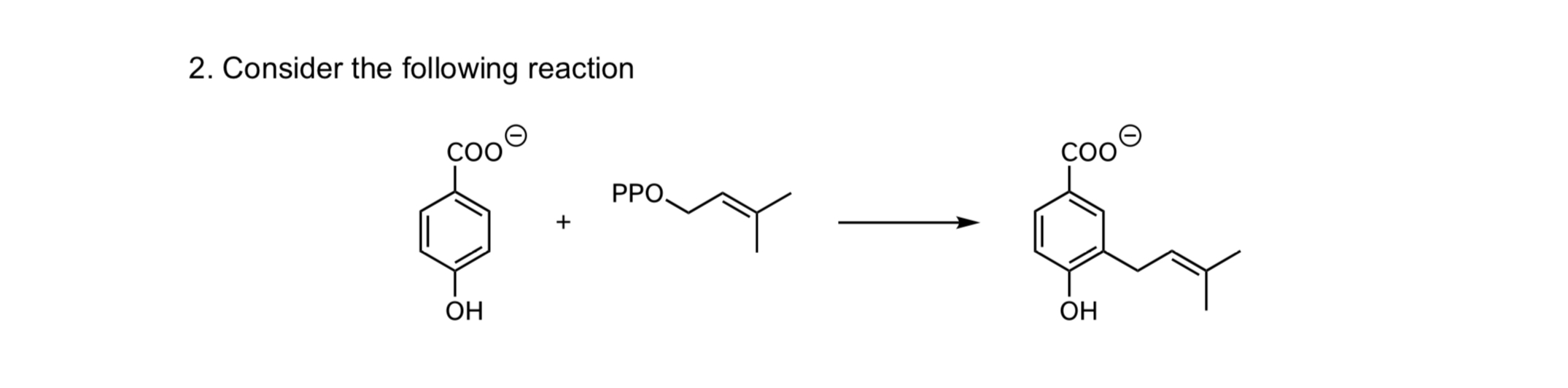
Solved Consider The Following Reaction Chegg Question: consider the following reaction: n2ha (l) 2h2o2 (1) > n2 (g) 4h20 (g) if ah\deg x = 530 kj at 298 k, then o the reaction is not spontaneous at any temperature. • the reaction is spontaneous at all temperatures. • the reaction will only be spontaneous at very low temperatures. Engineering chemical engineering chemical engineering questions and answers consider the following consecutive reactions taking place in an ideal batch reactor. please note the rate limiting step is abbreviated as rls. a s 1⇌as 1 b s 1⇌bs 1 2as 1 bs 1→cs 1 2d 2s 1 (rls) c s 1⇌cs 1 assuming elementary kinetics for the rate limiting step, derive the rate equation for the.
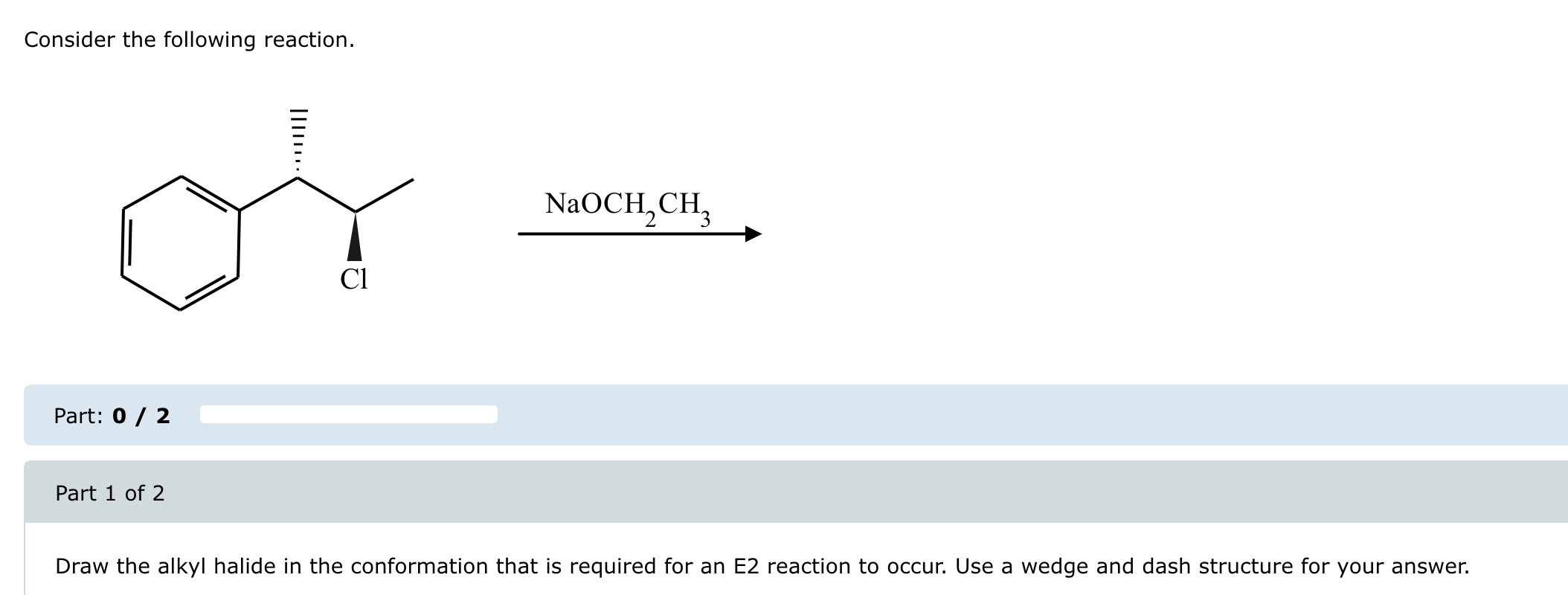
Solved Consider The Following Reaction Chegg Question: consider the following reaction: a (g) b (g)⇌2c (g),kc=1.4×10−5 when this reaction comes to equilibrium, will the concentrations of the reactants or products be greater? does the answer to this question depend on the initial concentrations of the reactants and products?. Our expert help has broken down your problem into an easy to learn solution you can count on. here’s the best way to solve it. solution : part a note : ★equilibrium constant only depends upon the … not the question you’re looking for? post any question and get expert help quickly. Study with quizlet and memorize flashcards containing terms like consider the following diagrams which show the progress for the reaction a (blue) ⇌ b (red). the equilibrium constant (k) for this reaction is 0.8. at which point does the reaction reach equilibrium?. Study with quizlet and memorize flashcards containing terms like consider the reaction: c3h8 (g) 5o2 (g) > 3co2 (g) 4h2o (g), where h= 531 kcal. which statement concerning this reaction is true , which bond is the strongest?, a chemical reaction requires 31.39 kj.
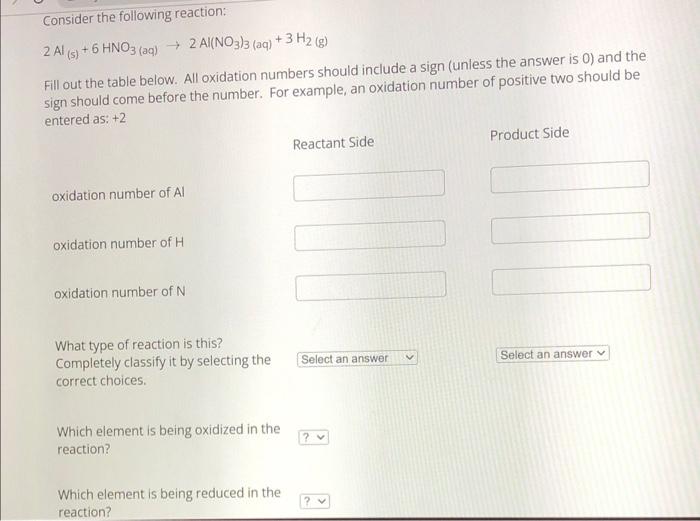
Solved Consider The Following Reaction Chegg Study with quizlet and memorize flashcards containing terms like consider the following diagrams which show the progress for the reaction a (blue) ⇌ b (red). the equilibrium constant (k) for this reaction is 0.8. at which point does the reaction reach equilibrium?. Study with quizlet and memorize flashcards containing terms like consider the reaction: c3h8 (g) 5o2 (g) > 3co2 (g) 4h2o (g), where h= 531 kcal. which statement concerning this reaction is true , which bond is the strongest?, a chemical reaction requires 31.39 kj. Consider the following redox reaction: ag⁺ (aq) fe²⁺ (aq) ⇌ ag (s) fe³⁺ (aq). how many moles of electrons are exchanged?. Define an elementary reaction, and state how it differs from an ordinary net chemical reaction. sketch out an activation energy diagram for a multistep mechanism involving a rate determining step, and relate this to the activation energy of the overall reaction. The nernst equation is given by: e cell =e cello − n0.0591 logq where e cello is the standard cell potential, n is the number of moles of electrons transferred, and q is the reaction quotient. step by step solution: step 1 identify the number of electrons transferred in the reaction. here, n =4. step 2 write the expression for the reaction.

Solved Consider The Following Reaction Chegg Consider the following redox reaction: ag⁺ (aq) fe²⁺ (aq) ⇌ ag (s) fe³⁺ (aq). how many moles of electrons are exchanged?. Define an elementary reaction, and state how it differs from an ordinary net chemical reaction. sketch out an activation energy diagram for a multistep mechanism involving a rate determining step, and relate this to the activation energy of the overall reaction. The nernst equation is given by: e cell =e cello − n0.0591 logq where e cello is the standard cell potential, n is the number of moles of electrons transferred, and q is the reaction quotient. step by step solution: step 1 identify the number of electrons transferred in the reaction. here, n =4. step 2 write the expression for the reaction.
