Solved Consider The Following Reaction H2so4 Predict The Product Of
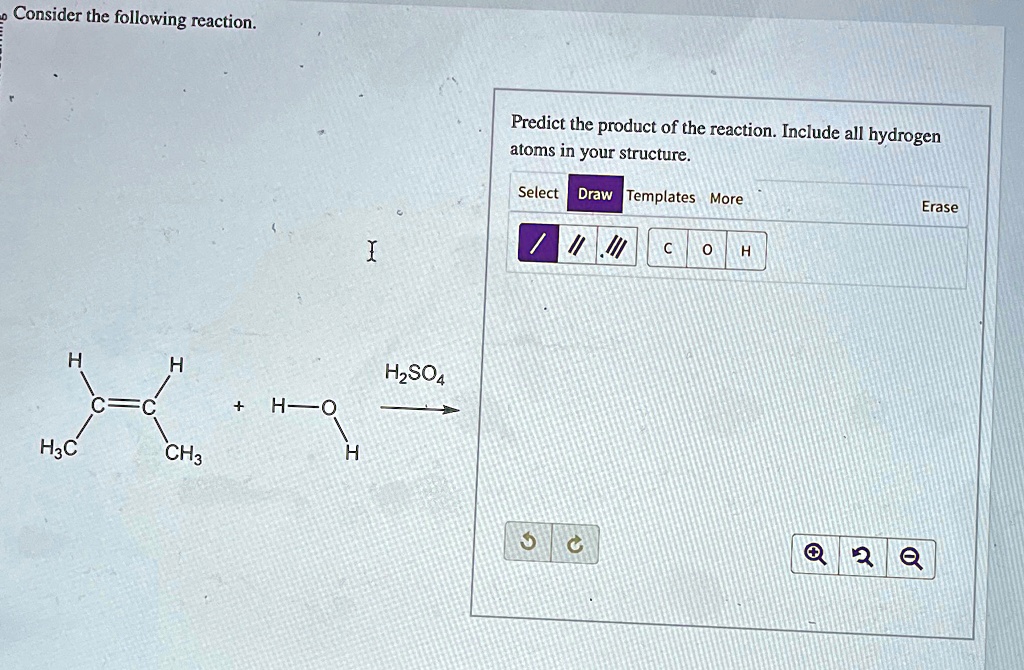
Consider The Following Reaction H H C C H O H3c Ch3 H H2so4 Predict There are 2 steps to solve this one. the structure of an alkene is given. it is made to react with h a 2 o in the presence of h a 2 so a 4. consider the following reaction. h2so4 predict the product of the reaction. include all hydrogen atoms in your structure. ec h h2c ch3 select draw rings more erase nh. not the question you’re looking for?. Interactive organic chemistry tools: predict reaction products, draw molecules, generate iupac names with ai, visualize mechanisms, and analyze chirality.
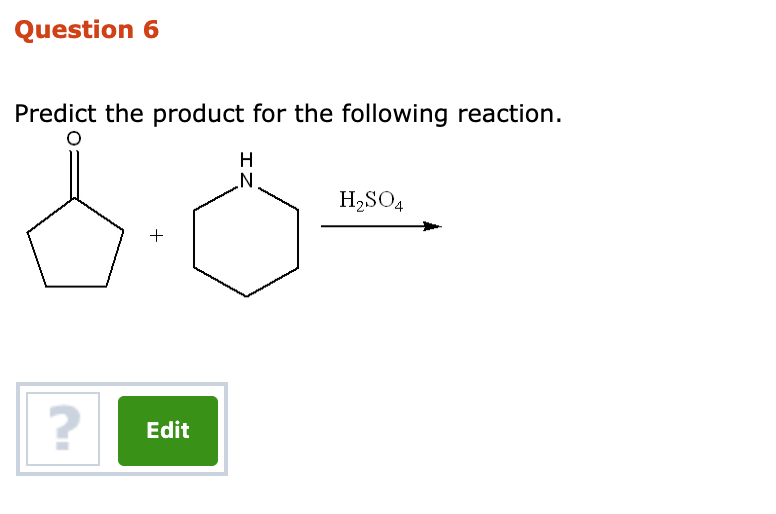
Solved Question 6 Predict The Product For The Following Chegg It isn’t possible to predict the products for all reactions. you must know how to find and balance ionic charge!. Write the products of the acid base reaction. in reaction (a), the products are hso4 and ch3cooh. in reaction (b), the products are ch3coo and (ch3)3nh . consider the equilibrium position. the strength of the acids and bases involved will determine the direction of the equilibrium. Sulfuric acid can react with water (h2o) to form hydronium ions (h3o ) and sulfate ions (so4^2 ). this reaction is known as the ionization or dissociation of sulfuric acid. h2so4 h2o > h3o so4^2 the reaction mechanism for this step involves the transfer of a proton from sulfuric acid to water, forming hydronium ions and sulfate ions.answer2. The major product of the reaction involving sulfuric acid (h₂so₄) with an alcohol is typically an alkene. this process is known as dehydration, where a water molecule is eliminated from the alcohol, resulting in the formation of a double bond.
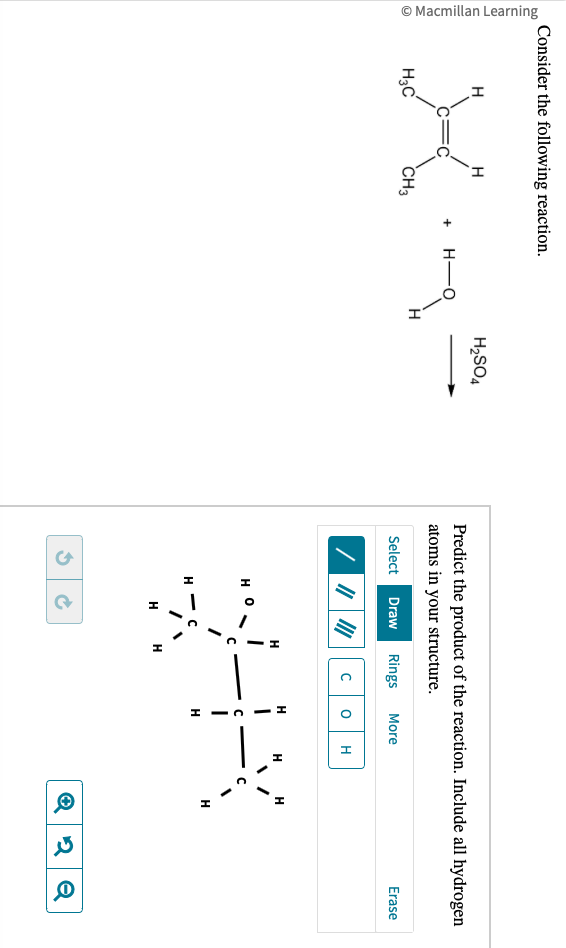
Solved Consider The Following Reaction Predict The Pro Sulfuric acid can react with water (h2o) to form hydronium ions (h3o ) and sulfate ions (so4^2 ). this reaction is known as the ionization or dissociation of sulfuric acid. h2so4 h2o > h3o so4^2 the reaction mechanism for this step involves the transfer of a proton from sulfuric acid to water, forming hydronium ions and sulfate ions.answer2. The major product of the reaction involving sulfuric acid (h₂so₄) with an alcohol is typically an alkene. this process is known as dehydration, where a water molecule is eliminated from the alcohol, resulting in the formation of a double bond. For example, solid iron reacting with aqueous sulfuric acid (h 2 so 4). in this reaction the question is whether iron will displace hydrogen and form hydrogen gas. consulting the activity series, we see that hydrogen is to the right of iron, meaning that the reaction is expected to occur. Consider how alkenes react with h to form a carbocation, allowing the h ion to join the double bond of the alkene, leaving a positively charged carbon atom. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and combustion reactions. We have a molecule with the structure h c≡c h. this is an alkyne with a triple bond between the two carbon atoms.answer3. the alkyne can act as a base and accept the proton from h2so4, forming a bond with one of the carbon atoms. so, the product of the reaction will be: $$\textbf {ch 3 c\equiv ch}$$.
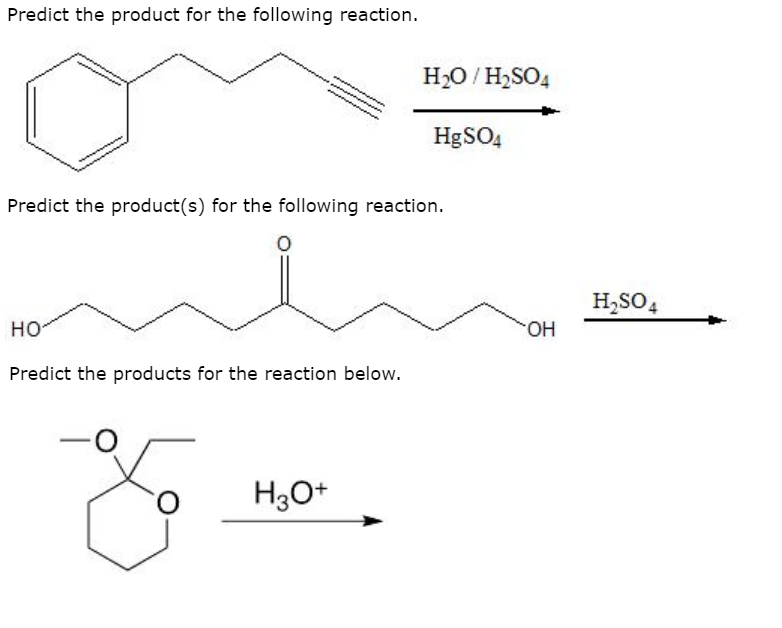
Solved Predict The Product For The Following Reaction H20 Chegg For example, solid iron reacting with aqueous sulfuric acid (h 2 so 4). in this reaction the question is whether iron will displace hydrogen and form hydrogen gas. consulting the activity series, we see that hydrogen is to the right of iron, meaning that the reaction is expected to occur. Consider how alkenes react with h to form a carbocation, allowing the h ion to join the double bond of the alkene, leaving a positively charged carbon atom. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and combustion reactions. We have a molecule with the structure h c≡c h. this is an alkyne with a triple bond between the two carbon atoms.answer3. the alkyne can act as a base and accept the proton from h2so4, forming a bond with one of the carbon atoms. so, the product of the reaction will be: $$\textbf {ch 3 c\equiv ch}$$.
Comments are closed.