Solved Consider The Following Reaction N2 G O2 G Chegg
Solved Consider The Following Reaction N2 G 02 G 2 No G Chegg Our expert help has broken down your problem into an easy to learn solution you can count on. here’s the best way to solve it. not the question you’re looking for? post any question and get expert help quickly. Consider the following reversible reaction at equilibrium: 2 no (g) o2 (g) > 2 no2 (g). if the pressure inside the reaction vessel is increased, the equilibrium will shift to the right.
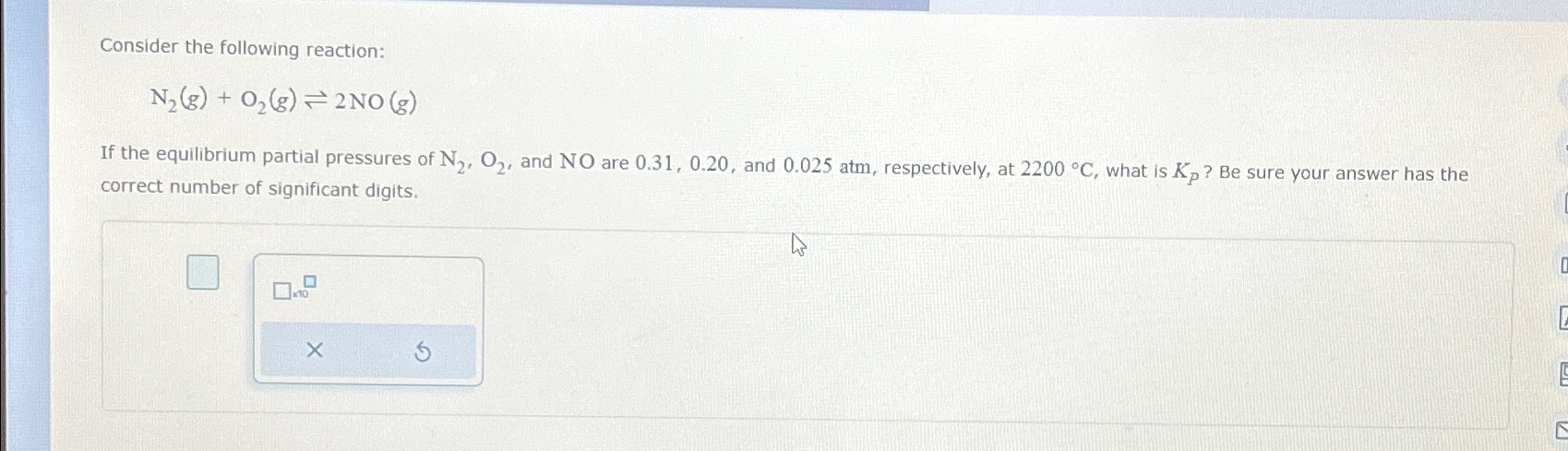
Solved Consider The Following Reaction N2 G O2 G 2no G If Chegg Given an initial concentration [n2o] of 0.50 m, a rate constant k = 3.4 × 10⁻³ s⁻¹, and a time t = 2.0 min (120 s), we can substitute these values into the formula to find the remaining concentration. N2 (g) o2 (g) → 2no (g), we can use hess's law, which states that the total enthalpy change for a reaction is the sum of the enthalpy changes for the individual steps. Since we are given the initial amount of no, we can calculate its initial concentration: [no]initial = 8.98 mol 2.0 l = 4.49 m since there are no initial amounts of n2 and o2, their initial concentrations are both zero: [n2]initial = [o2]initial = 0 m. Consider the reaction: 2 n2o ( g) >2 n2 ( g) o2 ( g) a. express the rate of the reaction in terms of the change in concentration of each of the reactants and products.

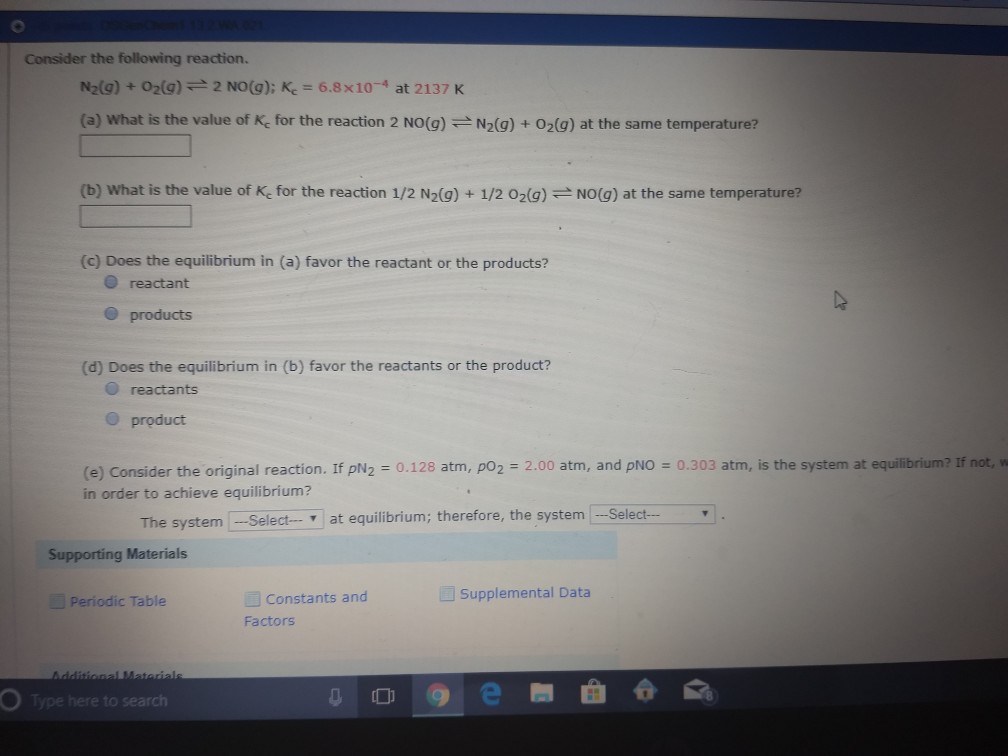
Solved 1 Consider The Following Reaction N2 G O2 G 2 Chegg Since we are given the initial amount of no, we can calculate its initial concentration: [no]initial = 8.98 mol 2.0 l = 4.49 m since there are no initial amounts of n2 and o2, their initial concentrations are both zero: [n2]initial = [o2]initial = 0 m. Consider the reaction: 2 n2o ( g) >2 n2 ( g) o2 ( g) a. express the rate of the reaction in terms of the change in concentration of each of the reactants and products. Identify that the given reaction 2 n o (g) → n 2 (g) o 2 (g) is the reverse of n 2 (g) o 2 (g) → 2 n o (g), so the equilibrium constant for the reverse reaction will be the reciprocal of the forward reaction's equilibrium constant. Adding more n 2 or removing o2 will shift the equilibrium of the reaction 2n 2 () ↔ o2() 2n 2() to the right. removing n 2 will not cause the equilibrium to shift to the right. therefore, the actions that shift the equilibrium to the right are options 1 and 2. For the given reaction, the stoichiometric coefficients indicate that for every 2 moles of n2o that decompose, 2 moles of n2 and 1 mole of o2 are produced. this relationship allows us to predict the rate of change in concentration of n2o based on the rate of o2 production. Consider the following reaction. n2 (g) o2 (g) < > 2 no (g) (a) if kc for this reaction is 1.5 10^−10, and a reaction system at equilibrium has an [n2] of 0.035 m and an [o2] of 0.035 m, what is the equilibrium concentration of no?.

Solved Consider The Following Reaction N2 G O 2 G Chegg Identify that the given reaction 2 n o (g) → n 2 (g) o 2 (g) is the reverse of n 2 (g) o 2 (g) → 2 n o (g), so the equilibrium constant for the reverse reaction will be the reciprocal of the forward reaction's equilibrium constant. Adding more n 2 or removing o2 will shift the equilibrium of the reaction 2n 2 () ↔ o2() 2n 2() to the right. removing n 2 will not cause the equilibrium to shift to the right. therefore, the actions that shift the equilibrium to the right are options 1 and 2. For the given reaction, the stoichiometric coefficients indicate that for every 2 moles of n2o that decompose, 2 moles of n2 and 1 mole of o2 are produced. this relationship allows us to predict the rate of change in concentration of n2o based on the rate of o2 production. Consider the following reaction. n2 (g) o2 (g) < > 2 no (g) (a) if kc for this reaction is 1.5 10^−10, and a reaction system at equilibrium has an [n2] of 0.035 m and an [o2] of 0.035 m, what is the equilibrium concentration of no?.

Solved Question 1 Consider The Following Reaction N2 G Chegg For the given reaction, the stoichiometric coefficients indicate that for every 2 moles of n2o that decompose, 2 moles of n2 and 1 mole of o2 are produced. this relationship allows us to predict the rate of change in concentration of n2o based on the rate of o2 production. Consider the following reaction. n2 (g) o2 (g) < > 2 no (g) (a) if kc for this reaction is 1.5 10^−10, and a reaction system at equilibrium has an [n2] of 0.035 m and an [o2] of 0.035 m, what is the equilibrium concentration of no?.
Comments are closed.