Solved Consider The Following Reaction Pb No3 2 Aq Cabr2 Aq
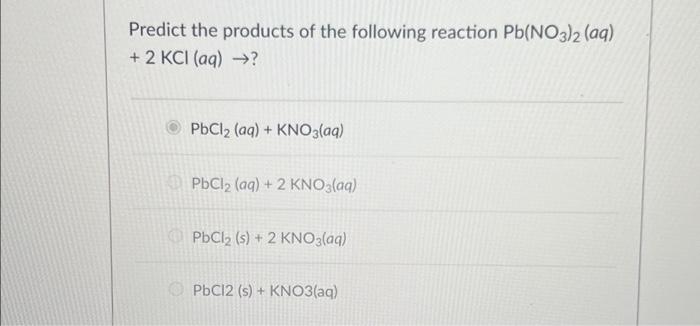
Solved Predict The Products Of The Following Reaction Chegg Our expert help has broken down your problem into an easy to learn solution you can count on. here’s the best way to solve it. the solution of the above question is given below with explanation. (b) pb 2 and … not the question you’re looking for? post any question and get expert help quickly. The number of atoms of each element on both sides of pb (no3)2 cabr2 = pbbr2 ca (no3)2 is equal which means that the equation is already balanced and no additional work is needed.

Solved Consider The Following Reaction Pb No3 2 Aq Kl Chegg General chem exam 5 considering the following precipitation reaction: pb (no3)2 (aq) 2ki (aq) → pbi2 (s) 2kno3 (aq) what is the correct complete ionic equation?. Identify the correct net ionic equation for the reaction that occurs when solutions of pb (no3)2 and nh4cl are mixed: pb (no3)2 (aq) 2nh4cl (aq) → pbcl2 (s) 2nh4no3 (aq). In this reaction, the spectator ions are potassium (k ) and nitrate (no3 ) 😉 still have questions? click here 👆 to get an answer to your question ️ consider the following precipitation reaction: pb (no 3) 2 (aq) 2ki (aq)to pbi 2 (s) 2kno 3 (aq). which ions are. To write the complete ionic equation for the given precipitation reaction, let's first understand the reaction and then break down the compounds into their ionic forms. the given chemical reaction is: pb (no3)2(aq) 2ki(aq) → pbi2(s) 2kno3(aq) here's how you can approach this: (lead (ii) nitrate) is a soluble ionic compound in aqueous solution.

Solved Example Pb No3 2 Aq 2 Kl Aq 2 Kno3 Aq Chegg In this reaction, the spectator ions are potassium (k ) and nitrate (no3 ) 😉 still have questions? click here 👆 to get an answer to your question ️ consider the following precipitation reaction: pb (no 3) 2 (aq) 2ki (aq)to pbi 2 (s) 2kno 3 (aq). which ions are. To write the complete ionic equation for the given precipitation reaction, let's first understand the reaction and then break down the compounds into their ionic forms. the given chemical reaction is: pb (no3)2(aq) 2ki(aq) → pbi2(s) 2kno3(aq) here's how you can approach this: (lead (ii) nitrate) is a soluble ionic compound in aqueous solution. A more complex problem: the common ion effect calculating the solubility of pb (io 3) 2 in deionized water is a straightforward problem because the solid’s dissolution is the only source of pb 2 and \ (\text {io} 3^ \). but what if we add pb (io 3) 2 to a solution of 0.10 m pb (no 3) 2? before we set‐up and solve this problem algebraically, think about the system’s chemistry and decide. Lead (ii) nitrate [pb (no₃)₂] and calcium bromide [cabr₂] are the reactants in this chemical reaction. both compounds are dissolved in water, forming aqueous solutions denoted by (aq). This method separates the reaction into two half reactions – one for oxidation and one for reduction. each half reaction is balanced separately and then combined. There are 2 steps to solve this one. the blank should be filled with "2pbi₂ (aq)." so, the balanced reaction would be: not the question you’re looking for? post any question and get expert help quickly.

Solved Classify Each Chemical Reaction Reaction Pb No3 Chegg A more complex problem: the common ion effect calculating the solubility of pb (io 3) 2 in deionized water is a straightforward problem because the solid’s dissolution is the only source of pb 2 and \ (\text {io} 3^ \). but what if we add pb (io 3) 2 to a solution of 0.10 m pb (no 3) 2? before we set‐up and solve this problem algebraically, think about the system’s chemistry and decide. Lead (ii) nitrate [pb (no₃)₂] and calcium bromide [cabr₂] are the reactants in this chemical reaction. both compounds are dissolved in water, forming aqueous solutions denoted by (aq). This method separates the reaction into two half reactions – one for oxidation and one for reduction. each half reaction is balanced separately and then combined. There are 2 steps to solve this one. the blank should be filled with "2pbi₂ (aq)." so, the balanced reaction would be: not the question you’re looking for? post any question and get expert help quickly.
Comments are closed.