Solved Consider The Following Three Molecules I Ii And Chegg
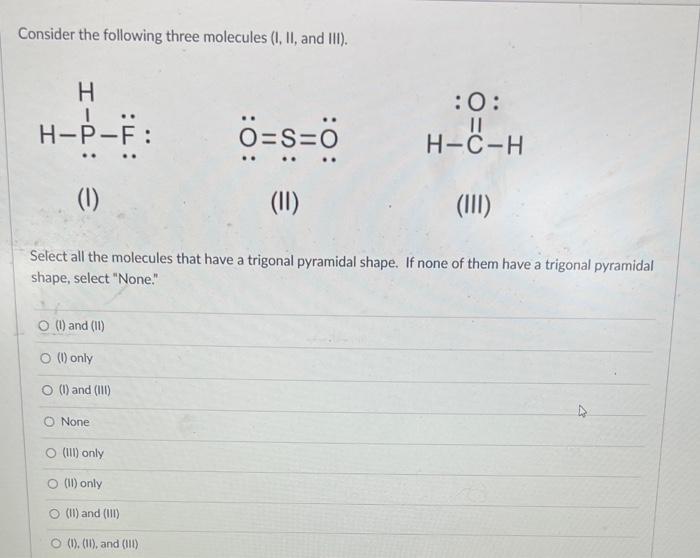
Solved Consider The Following Three Molecules I Ii And Chegg Iii. no select all the molecules that violate the octet rule. here’s the best way to solve it. Consider the intermolecular forces present in a pure sample of each of the following compounds: ch₃cl and ccl₄. identify the intermolecular forces that these compounds have in common.
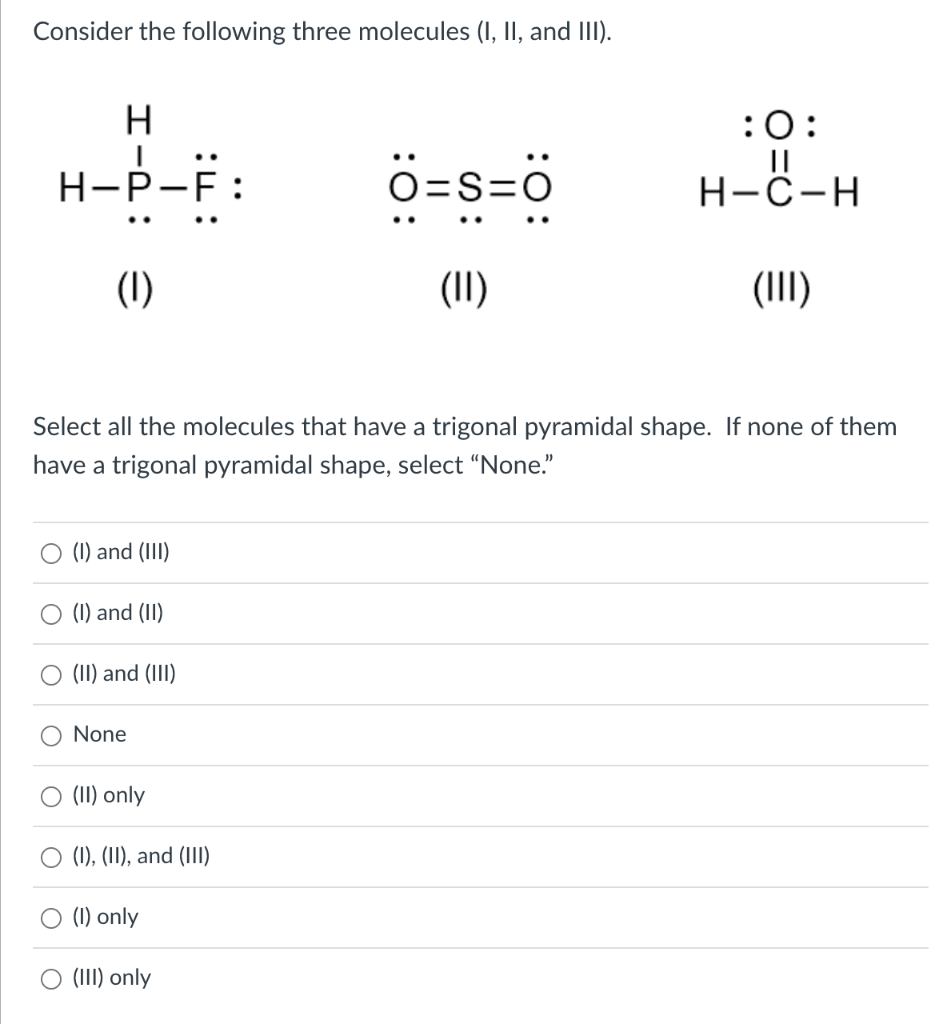
Solved Consider The Following Three Molecules I Ii And Chegg Since the specific details of molecules (i), (ii), and (iii) were not provided, you would need to apply the criteria for a trigonal pyramidal shape to each molecule based on its actual structure to make the correct selection. Consider the following three molecules (i, ii, and iii). (i) (ii) (iii) select all the molecules that have a trigonal pyramidal shape. if none of them have a trigonal pyramidal shape, select "none." answered: 1 week ago. Each problem printed in the text is reproduced in this manual, followed by a worked out solution. if a figure or table accompanies a problem in the text, it is also reproduced here. included within a solution may be an additional figure or table that does not appear in the text. Consider three molecules: a, b, and c. molecule a has a hybridization of s p 3. molecule b has two more effective pairs (electron pairs around the central atom) than molecule a. molecule c consists of two σ bonds and two π bonds.
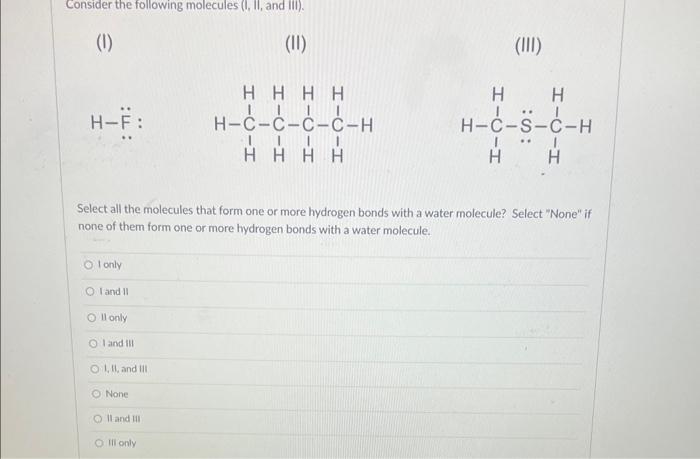
Solved Consider The Following Molecules I Ii And Iii Chegg Each problem printed in the text is reproduced in this manual, followed by a worked out solution. if a figure or table accompanies a problem in the text, it is also reproduced here. included within a solution may be an additional figure or table that does not appear in the text. Consider three molecules: a, b, and c. molecule a has a hybridization of s p 3. molecule b has two more effective pairs (electron pairs around the central atom) than molecule a. molecule c consists of two σ bonds and two π bonds. For the following questions, choose all the answers that are correct: which of the following molecules are capable of hydrogen bonding with themselves?. Question: consider the following molecules (i, ii, and iii). (1) (ii) (iii) h−f¨: select all the molecules that form one or more hydrogen bonds with a water molecule?. To calculate the heat that is evolved in converting 1.00 mol of steam at 160.0 ∘c to ice at 45.0 ∘c use the three following equations to solve for Δq and Δh values:. Instant answer step 1 2 i. bf3: boron has 3 valence electrons, and it forms 3 bonds with 3 f atoms. so, it has only 6 electrons around it, which violates the octet rule. ii. chbr3: carbon has 4 valence electrons, and it forms 4 bonds with 1 h and 3 br atoms. so, it has a complete octet (8 electrons). iii.

Solved Consider The Following Molecules I Ii And Iii Chegg For the following questions, choose all the answers that are correct: which of the following molecules are capable of hydrogen bonding with themselves?. Question: consider the following molecules (i, ii, and iii). (1) (ii) (iii) h−f¨: select all the molecules that form one or more hydrogen bonds with a water molecule?. To calculate the heat that is evolved in converting 1.00 mol of steam at 160.0 ∘c to ice at 45.0 ∘c use the three following equations to solve for Δq and Δh values:. Instant answer step 1 2 i. bf3: boron has 3 valence electrons, and it forms 3 bonds with 3 f atoms. so, it has only 6 electrons around it, which violates the octet rule. ii. chbr3: carbon has 4 valence electrons, and it forms 4 bonds with 1 h and 3 br atoms. so, it has a complete octet (8 electrons). iii.

Solved Consider The Following Molecules I Ii And Iii Chegg To calculate the heat that is evolved in converting 1.00 mol of steam at 160.0 ∘c to ice at 45.0 ∘c use the three following equations to solve for Δq and Δh values:. Instant answer step 1 2 i. bf3: boron has 3 valence electrons, and it forms 3 bonds with 3 f atoms. so, it has only 6 electrons around it, which violates the octet rule. ii. chbr3: carbon has 4 valence electrons, and it forms 4 bonds with 1 h and 3 br atoms. so, it has a complete octet (8 electrons). iii.
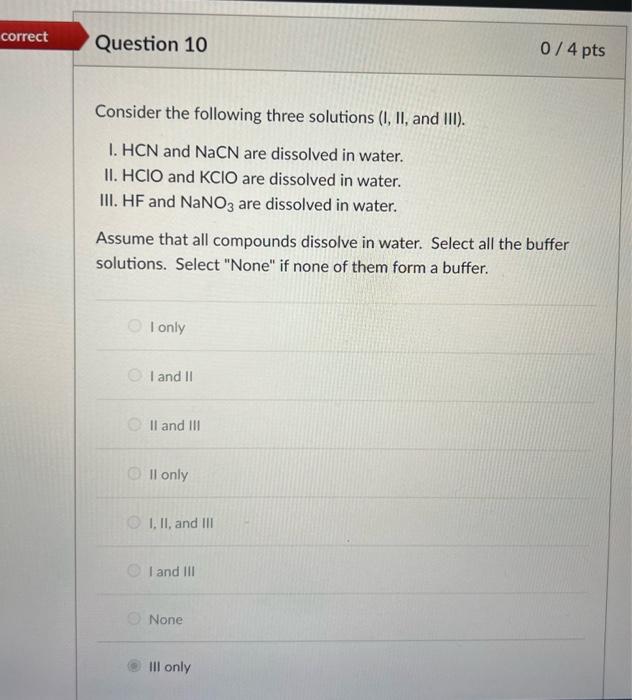
Solved Consider The Following Molecules I Ii And Iii Chegg
Comments are closed.