Solved Consider The Following Two Reactions N2 G O2 Chegg
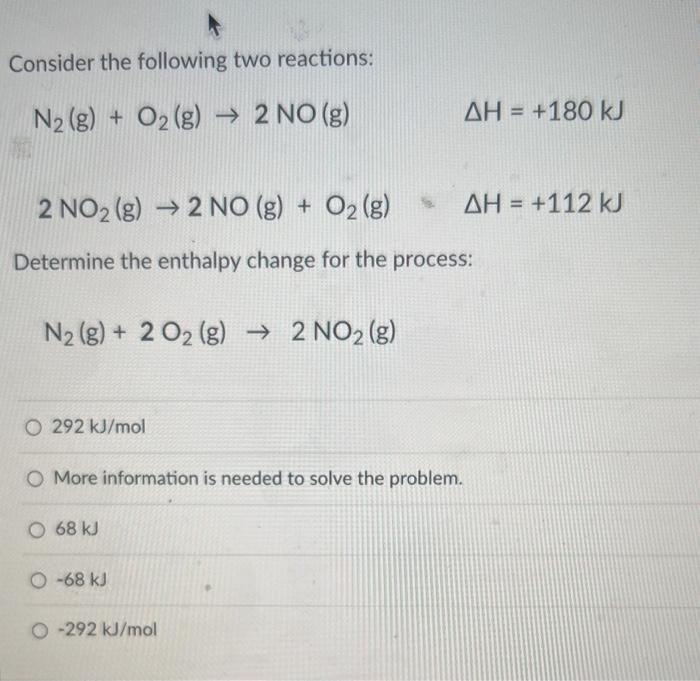
Solved Consider The Following Two Reactions N2 G O2 Chegg Here’s the best way to solve it. apply hess's law to combine the given reactions to find the enthalpy change for the desired reaction. not the question you’re looking for? post any question and get expert help quickly. N2 (g) o2 (g) → 2no (g), we can use hess's law, which states that the total enthalpy change for a reaction is the sum of the enthalpy changes for the individual steps.

Solved Consider The Following Reactions N2 G 2o2 Chegg Adding more n 2 or removing o2 will shift the equilibrium of the reaction 2n 2 () ↔ o2() 2n 2() to the right. removing n 2 will not cause the equilibrium to shift to the right. therefore, the actions that shift the equilibrium to the right are options 1 and 2. Calculate the heat (kj) released to the surroundings when 38.5 g of o2 (g) reacts with excess co. the specific heat of liquid bromine (br2) is 0.226 j g k. how much heat (j) is required to raise the temperature of 10.0 ml of bromine from 25.00 °c to 27.30 °c? the density of liquid bromine: 3.12 g ml. Solved: consider the reactions: n2 (g) o2 (g) ⇌ 2no (g) n2 (g) 2o2 (g) ⇌ n2o4 (g) how would you expect the values of Δs for these reactions to compare?. Determine and for the reaction using standard enthalpy and entropy values from a data table. this video solution was recommended by our tutors as helpful for the problem above. was this helpful? here are the essential concepts you must grasp in order to answer the question correctly.
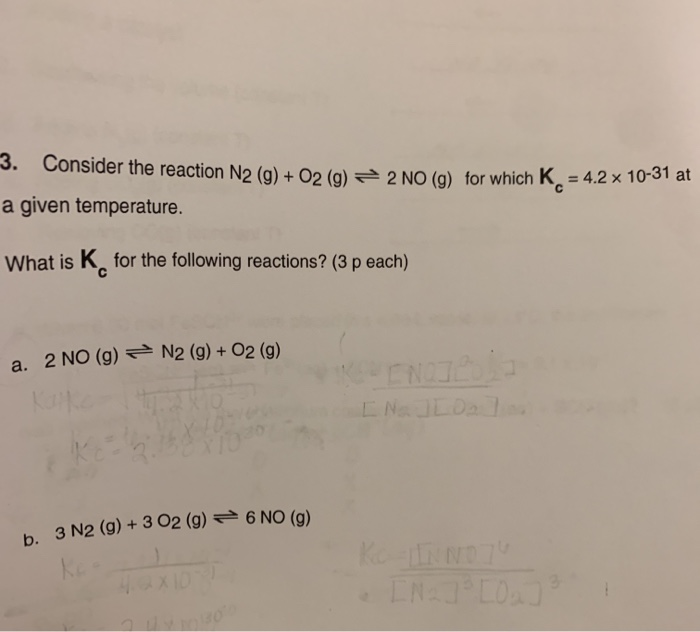
Solved 3 Consider The Reaction N2 G O2 G 2 No G For Chegg Solved: consider the reactions: n2 (g) o2 (g) ⇌ 2no (g) n2 (g) 2o2 (g) ⇌ n2o4 (g) how would you expect the values of Δs for these reactions to compare?. Determine and for the reaction using standard enthalpy and entropy values from a data table. this video solution was recommended by our tutors as helpful for the problem above. was this helpful? here are the essential concepts you must grasp in order to answer the question correctly. Given an initial concentration [n2o] of 0.50 m, a rate constant k = 3.4 × 10⁻³ s⁻¹, and a time t = 2.0 min (120 s), we can substitute these values into the formula to find the remaining concentration. Here’s the best way to solve it. answer entha … not the question you’re looking for? post any question and get expert help quickly. To find this, we can rearrange the given reactions to match the desired reaction and then add the enthalpy changes. rearrange reaction 2 to get 2no2 (g) → 2no (g) o2 (g) with Δh = 114.2 kj (the sign changes because the reaction is reversed). This problem has been solved! you'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Comments are closed.