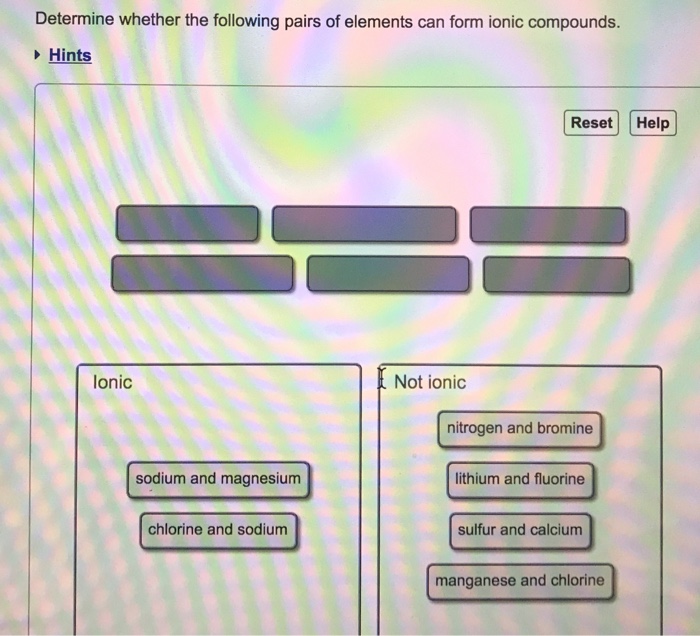
Solved Determine Whether The Following Pairs Of Elements Chegg There are 4 steps to solve this one. ionic compounds: generally formed between metals and non metals. in thes determine whether the following compounds are ionic or molecular: naf,hf(g),mgcl2,fecl3,o2, n2o, cao,hcl(g),lif,f2o,baf2,pcl5,al2o3,brf4,nacn,so2 what types of elements are bonded in ionic compounds?. Determine whether each of the following compounds would be best represented by an ionic or a covalent lewis structure. use lewis theory to determine the formula for the compound that forms from mg and br.
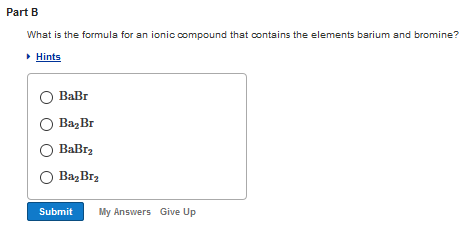
Solved Determine Whether The Following Pairs Of Elements Can Chegg Classify compounds as ionic or molecular. if they are ionic, determine whether the metal forms only one type of ion or more than one type of ion. a) ionic. fe transitional metal forms more than one type of ion, cl nonmetal. b) ionic. k metal forms only one type of ion (part of alkali metals family). so₄ polyatomic ion. c) molecular. Determine whether the following pairs of elements can form ionic compounds. what is the formula for an ionic compound that contains the elements magnesium and chlorine? we have an expert written solution to this problem! sort the following iron compounds by whether the cation is iron (ii) or iron (iii). There are 2 steps to solve this one. two basic categories of chemical compounds, ionic and covalent, each have unique bonding traits and not the question you’re looking for? post any question and get expert help quickly. There are 2 steps to solve this one. 1. the first compound a is ionic compound. 1. determine whether each of the compounds is ionic or covalent. 2. look at the results carefully. are there any patterns that you have observed in the property of solubility? explain. 3. similarly, look at the other properties.
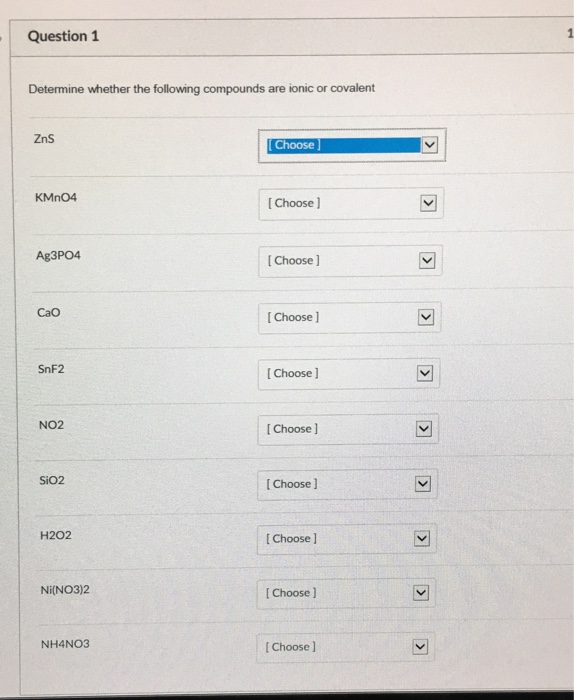
Solved Determine Whether The Following Compounds Are Ionic Chegg There are 2 steps to solve this one. two basic categories of chemical compounds, ionic and covalent, each have unique bonding traits and not the question you’re looking for? post any question and get expert help quickly. There are 2 steps to solve this one. 1. the first compound a is ionic compound. 1. determine whether each of the compounds is ionic or covalent. 2. look at the results carefully. are there any patterns that you have observed in the property of solubility? explain. 3. similarly, look at the other properties. Using the periodic table, predict whether the following chlorides are ionic or covalent: sicl 4, pcl 3, cacl 2, cscl, cucl 2, and crcl 3. for each of the following compounds, state whether it is ionic or covalent. if it is ionic, write the symbols for the ions involved:. We're going to begin by defining what each of these terms represent. so for ionic compounds, we want to recall that, that's describing the transfer of electrons between two species. either a metal or poly atomic ion and a non metal or poly atomic ion. Determine whether the following compounds are covalent or ionic and give them their proper names. 1. ba (no3)2 2. co 3. pci 4. ki 5. cfa 6. mgo 7. cuzs 8. so2 9. nci 10.xef6. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. Study with quizlet and memorize flashcards containing terms like write the following formula for the ionic compound: copper (i) chlorate, write the following formula for the ionic compound: potassium permanganate, write the following formula for the ionic compound: lead (ii) chromate and more.

Solved State Whether The Following Compounds Are Ionic Or Chegg Using the periodic table, predict whether the following chlorides are ionic or covalent: sicl 4, pcl 3, cacl 2, cscl, cucl 2, and crcl 3. for each of the following compounds, state whether it is ionic or covalent. if it is ionic, write the symbols for the ions involved:. We're going to begin by defining what each of these terms represent. so for ionic compounds, we want to recall that, that's describing the transfer of electrons between two species. either a metal or poly atomic ion and a non metal or poly atomic ion. Determine whether the following compounds are covalent or ionic and give them their proper names. 1. ba (no3)2 2. co 3. pci 4. ki 5. cfa 6. mgo 7. cuzs 8. so2 9. nci 10.xef6. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. Study with quizlet and memorize flashcards containing terms like write the following formula for the ionic compound: copper (i) chlorate, write the following formula for the ionic compound: potassium permanganate, write the following formula for the ionic compound: lead (ii) chromate and more.
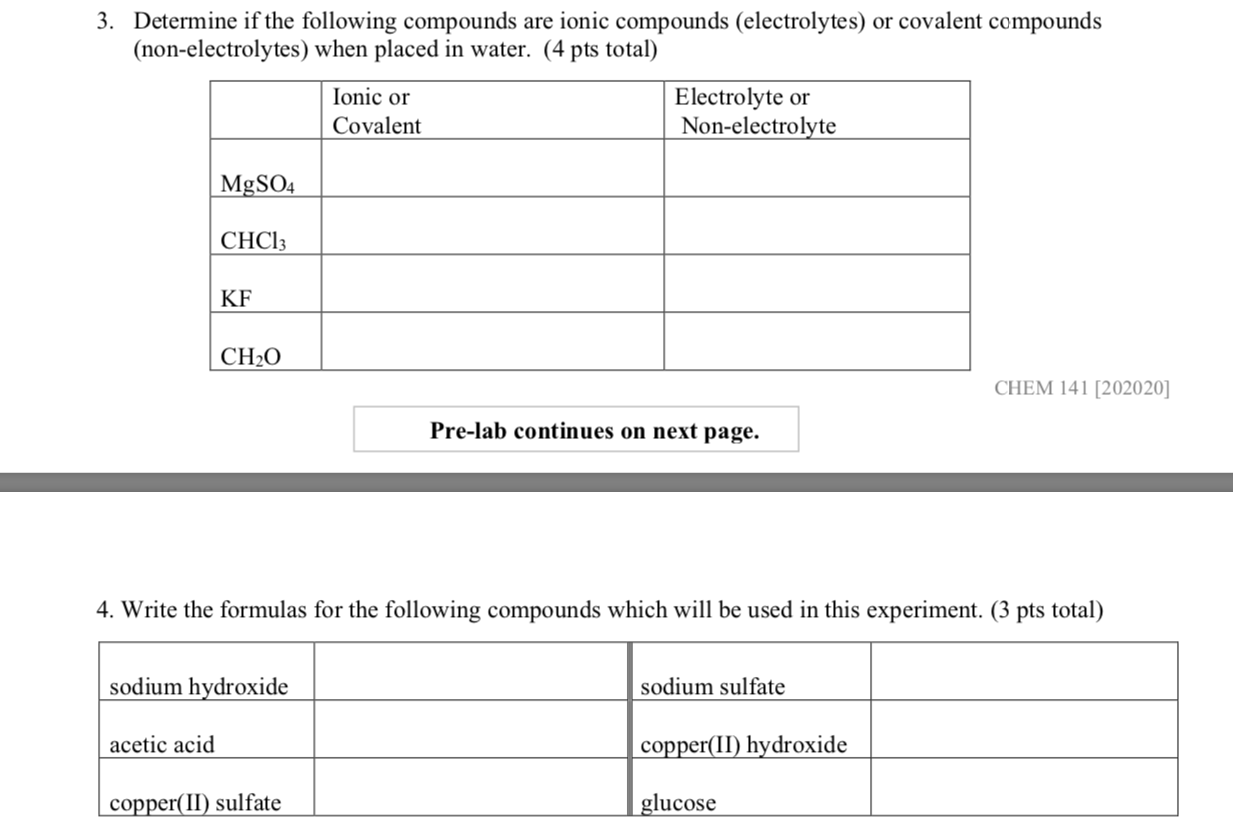
Solved 3 Determine If The Following Compounds Are Ionic Chegg Determine whether the following compounds are covalent or ionic and give them their proper names. 1. ba (no3)2 2. co 3. pci 4. ki 5. cfa 6. mgo 7. cuzs 8. so2 9. nci 10.xef6. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. Study with quizlet and memorize flashcards containing terms like write the following formula for the ionic compound: copper (i) chlorate, write the following formula for the ionic compound: potassium permanganate, write the following formula for the ionic compound: lead (ii) chromate and more.
