Solved Draw The Two Isomers Of Mn Nh3 5 No2 2 Draw A Chegg

Solved Draw Two Linkage Isomers Of Mn Nh3 5 No2 2 Chegg Our expert help has broken down your problem into an easy to learn solution you can count on. question: draw the two isomers of [mn (nh3)5 (no2)]2 draw a molecule on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. d c hp 3d exp." Çont . hhh h c n o s. Draw two linkage isomers of [mn(nh3)5(no2)]2 . linkage isomers are a type of coordination isomerism that occurs when ligands can coordinate to a central metal ion through different atoms. in the case of [mn (nh3)5 (no2)]2 , two possible linkage isomers can be formed.

Solved Draw The Two Isomers Of Mn Nh3 5 No2 2 Draw A Chegg Calculate the output impedance of the two transistor diode connected circuit shown in fig. p6.18 using smallsignal analysis. assume both transistors are in the active region, ignore the body effect, and assume gm1=gm2, rds1=rds2, and gmrds>>1. In the given complex, the ligand that can form linkage isomers is the nitrite ion (no2 ). it can bind to the metal center through either the nitrogen atom (n no2) or the oxygen atom (o no2).answerstep 2: draw the two linkage isomers. Find step by step chemistry solutions and your answer to the following textbook question: draw two linkage isomers of $ [mn (nh 3) 5 (no 2)]^ {2 }$. As we can see here, the nh3 acted as a weak field ligand in [mn (nh3)6] 2 because the complex has a weak field splitting which resulted in 5 unpaired electrons.

Solved Draw Two Linkage Isomers Of Mn Nh 3 5 No 2 2 Chegg Find step by step chemistry solutions and your answer to the following textbook question: draw two linkage isomers of $ [mn (nh 3) 5 (no 2)]^ {2 }$. As we can see here, the nh3 acted as a weak field ligand in [mn (nh3)6] 2 because the complex has a weak field splitting which resulted in 5 unpaired electrons. In the exercise [mn (nh3)5 (no2)]2 , the no2 can attach via the nitrogen to form a 'nitro' isomer, or via an oxygen to form a 'nitrito' isomer. this subtle change can impact properties like solubility, reactivity, and color. The coordination compound [mn (nh3)5 (no2)]2 can exhibit linkage isomerism due to the presence of the nitrite ligand (no2 ), coordinate through either the nitrogen atom (n bound) or the oxygen atom (o bound). Draw two linkage isomers of [mn (nh3)5 (no2)]2 . i know how to draw them separately but they need to be connected with bonds. no formal charges or lone pairs are needed. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. Linkage isomerism arises when the ligand has two atoms with lone pair of electrons and either of the donor atoms can coordinate with the metal to form coordinate covalent bonds with the metal.
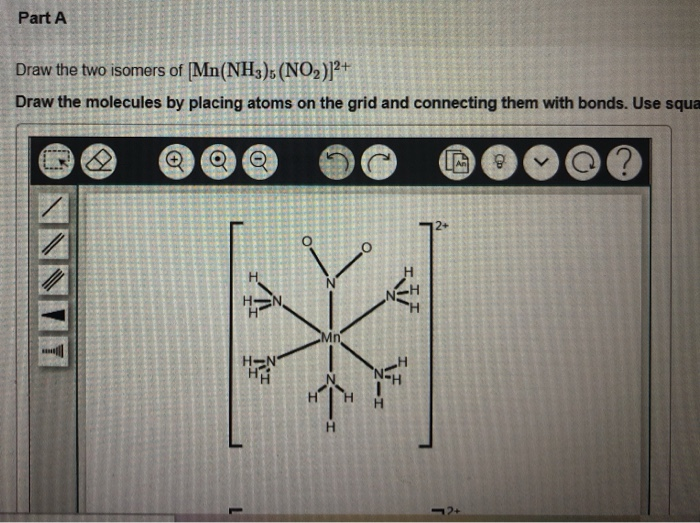
Solved Part A Draw The Two Isomers Of Mn Nh3 No2 2 Chegg In the exercise [mn (nh3)5 (no2)]2 , the no2 can attach via the nitrogen to form a 'nitro' isomer, or via an oxygen to form a 'nitrito' isomer. this subtle change can impact properties like solubility, reactivity, and color. The coordination compound [mn (nh3)5 (no2)]2 can exhibit linkage isomerism due to the presence of the nitrite ligand (no2 ), coordinate through either the nitrogen atom (n bound) or the oxygen atom (o bound). Draw two linkage isomers of [mn (nh3)5 (no2)]2 . i know how to draw them separately but they need to be connected with bonds. no formal charges or lone pairs are needed. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. Linkage isomerism arises when the ligand has two atoms with lone pair of electrons and either of the donor atoms can coordinate with the metal to form coordinate covalent bonds with the metal.
Comments are closed.