Solved Energy Diagrams For Two Reactions Are Shown What Is Chegg
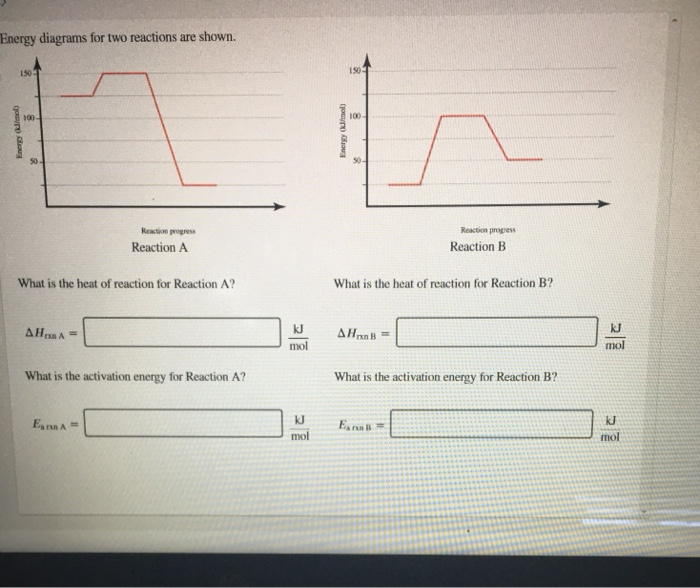
Solved Energy Diagrams For Two Reactions Are Shown 150 Chegg Question: energy diagrams for two reactions are shown. what is the heat of reaction for reaction a ? what is the heat of reaction for reaction b ? Δhrxna=molkjΔhrxnb=molkj what is the activation energy for reaction a? what is the activation energy for reaction b? there are 3 steps to solve this one. energy diagrams for two reactions are shown. To find the heat of reaction for two different reactions (reaction a and reaction b), you need to look at the energy levels of the reactants and products on the energy diagrams provided. for each reaction, identify the energy of both the reactants and products.
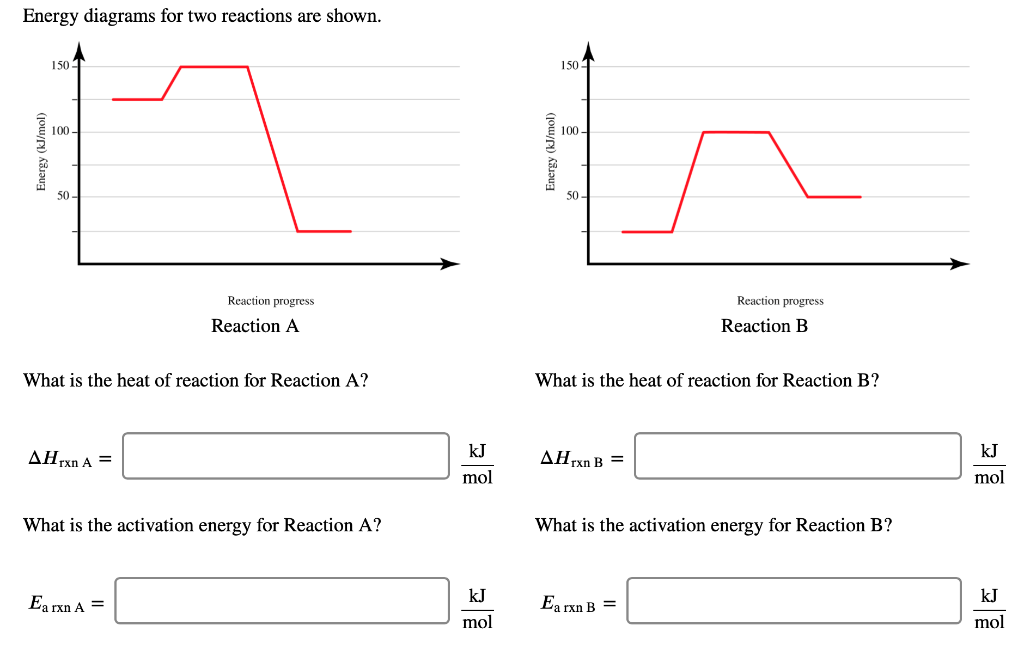
Solved Energy Diagrams For Two Reactions Are Shown 1501 Chegg Reaction a the heat of reaction for reaction a is the difference in energy between the products and the reactants. in this case, the energy of the products is 200 kj and the energy of the reactants is 100 kj. In an energy diagram, you can find ea by subtracting the energy of the reactants from the energy of the transition state. the larger the ea, the more energy is required for the reaction to occur. Draw a reaction coordinate diagram for a reaction that is exergonic (exothermic), has two intermediates, and the rate determining step is the second ts2 ts, ene rgy intermediate reactant product. Energy diagrams illustrate the potential energy changes during a chemical reaction. they depict the relationship between reactants, products, and the energy required to initiate the reaction.

Solved Consider The Two Energy Diagrams Shown Below A B Chegg Draw a reaction coordinate diagram for a reaction that is exergonic (exothermic), has two intermediates, and the rate determining step is the second ts2 ts, ene rgy intermediate reactant product. Energy diagrams illustrate the potential energy changes during a chemical reaction. they depict the relationship between reactants, products, and the energy required to initiate the reaction. 1. is the overall reaction, as shown, exothermic or endothermic? 2. which letter represents the activation energy for the forward reaction? 3. which letter represents the activation energy for the r everse reaction? 4. which letter represents the enthalpy change (∆h) for the forward reaction?. A reaction energy diagram visually represents the energy changes during a chemical reaction. it typically shows the energy of reactants and products, as well as the energy barriers (activation energies) that must be overcome for the reaction to proceed. It is the difference in energy between the reactants and the transition state. in reaction a, the activation energy is the difference between the energy of the reactants and the peak of the energy diagram. Step 1 let us solve for reaction a: a) Δ h r x n a = Δ h f i n a l − Δ h i n i t i a l form graph, we see that, Δ h i n i t i a l = 125 k j m o l and Δ h f i n a l = 25 k j m o l substituting the values now, we get, Δ h r x n a = 25 − 125 = − 100 k j m o l b) act.
Comments are closed.