Solved Exam 3 Calculate The Standard Enthalpy And Entropy Chegg
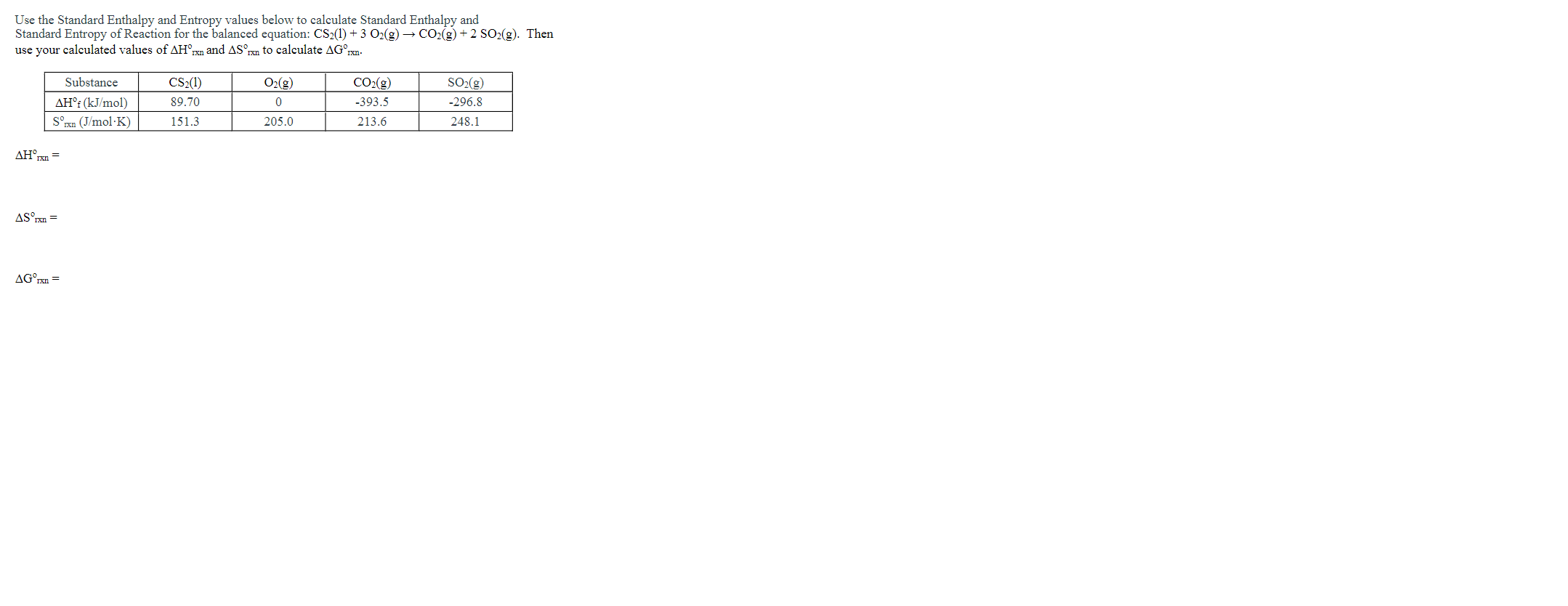
Solved Use The Standard Enthalpy And Entropy Values Below To Chegg Here’s the best way to solve it. exam 3. calculate the standard enthalpy and entropy of the following reactions: c&h. (1) 3h2 (g) = c6h12 (1) not the question you’re looking for? post any question and get expert help quickly. answer to exam 3. calculate the standard enthalpy and entropy. Find the standard molar enthalpy for the reaction c(s) 1⁄2 o2(g) → co(g) given that c(s) o2(g) → co2(g) ∆ho = 394 kj and co2(g) → co(g) 1⁄2 o2(g) ∆ho = 283 kj.
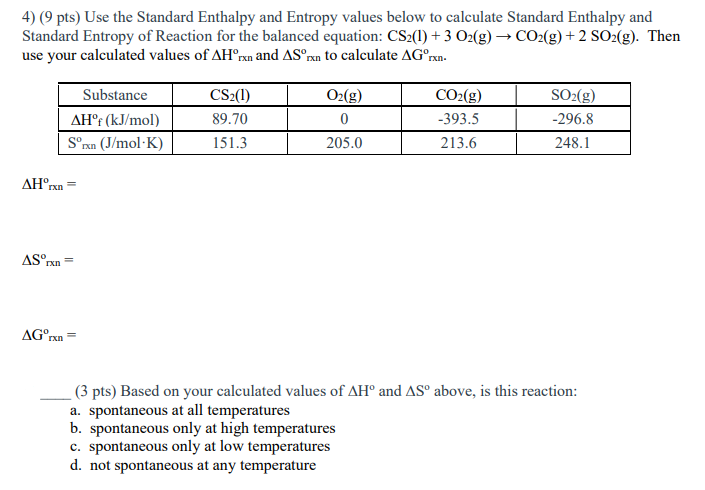
Solved 4 9 Pts Use The Standard Enthalpy And Entropy Chegg Go to standard state and standard enthalpy of formation for listing of standard enthalpy of formation and standard entrypy for more than 370 inorganic substances. 1) use the standard enthalpy and entropy values provided below to calculate the standard free energy change (Δg˚) for the vaporization of liquid h2o at room temperature (298 k). Entropy is a state function, so we can calculate values for a process using any path. this allows us to calculate the entropy change of a chemical reaction using standard entropies. There are 2 steps to solve this one. the standard enthalpy change of formation for lioh (s) is 487 kj mol . not the question you’re looking for? post any question and get expert help quickly. answer to 3.) use the standard entropy data in appendix g of.
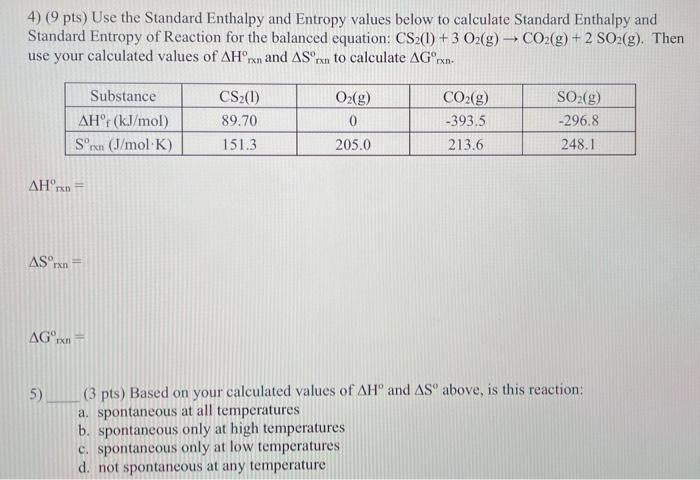
Solved 4 9 Pts Use The Standard Enthalpy And Entropy Chegg Entropy is a state function, so we can calculate values for a process using any path. this allows us to calculate the entropy change of a chemical reaction using standard entropies. There are 2 steps to solve this one. the standard enthalpy change of formation for lioh (s) is 487 kj mol . not the question you’re looking for? post any question and get expert help quickly. answer to 3.) use the standard entropy data in appendix g of. Using data of sm° from the appendix in your book, calculate the standard entropy change for each of the following reactions at 25°c. for each reaction, interpret the sign and magnitude of the reaction entropy. The standard enthalpy changes of combustion for the three substances in the equation are: c(s) h2(g) c5h12(l) mo calculate the standard enthalpy of formation of pentane. 3. use the values for standard enthalpy of formation to calculate the standard enthalpy change for the reaction: nh3(g) hcl(g) nh4cl(s). This page explains how to calculate entropy changes for different thermodynamic processes, such as isothermal, isobaric, isochoric, adiabatic changes, and phase transitions. (b) give the formula for calculating the standard enthalpy change of reaction, Δh r , using bond energies. (c) use the data given in table 3.1 to calculate the enthalpy change for the hydration of ethene to form ethanol.
Comments are closed.