Solved For The Following Free Energy Diagram Select All Of Chegg
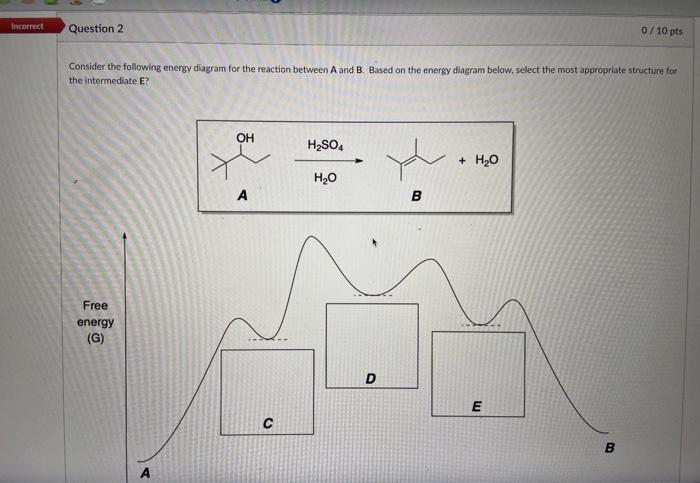
Solved Consider The Following Energy Diagram For The Chegg Step 1 given: free energy diagram of a chemical reaction. in this free energy diagram points a, b, c, d and e are present point a represe. Refer to the free energy diagrams below to answer the following questions. you may assume that the y axis is the same and directly comparable for all four reactions.
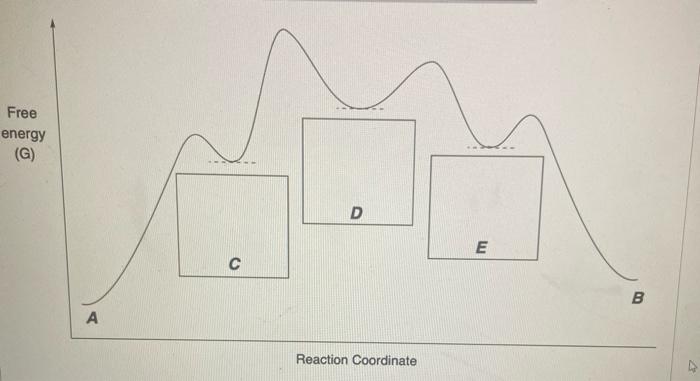
Solved Consider The Following Energy Diagram For The Chegg In this diagram, points b and d exhibit higher free energy, suggesting non equilibrium conditions. this analysis aids in predicting the direction of spontaneous reactions and the overall behavior of the system in response to changes in conditions. One side of each free energy curve will likely be lower than the other, this lower side should reside at the graph edge where the phase is more stable (see the ti curves, for example). all curve tangents should correspond to locations of changing phase stability on the binary phase diagram. On studocu you find all the lecture notes, summaries and study guides you need to pass your exams with better grades. Here’s the best way to solve it.
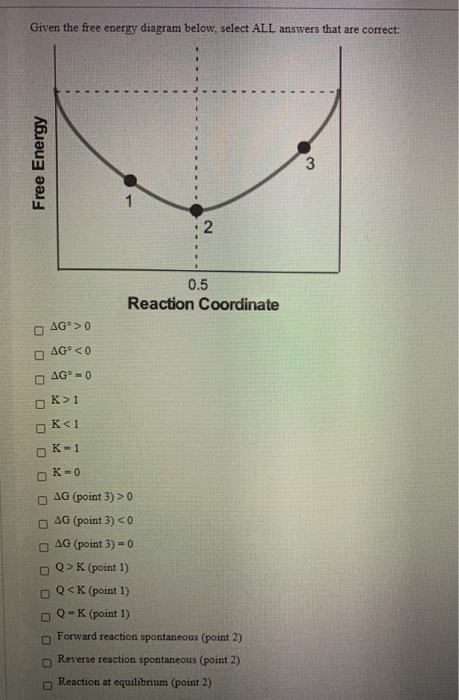
Solved Given The Free Energy Diagram Below Select All Chegg On studocu you find all the lecture notes, summaries and study guides you need to pass your exams with better grades. Here’s the best way to solve it. For a reaction for which ∆h = 64.2 kj mol and ∆s = 285 j mol・k, which of the following statements is true? a) the reaction is spontaneous only above 225 k. We can see the energy levels of the reactants, products, and transition state of a chemical reaction. we can also see the activation energy required for the reaction to occur and the overall change in free energy (Δg) for the reaction. Knowledge of either the standard free energy change or the equilibrium constant for a reaction allows for the calculation of the other. the following two sample problems illustrate each case. On the diagram above draw a line indicating the energy o f the activated complex. label this line “f”. draw. the symbol for the activated complex in the appropriate loc ation.
Comments are closed.