Solved For The Following Systems At Equilibrium A 2nocl G Chegg
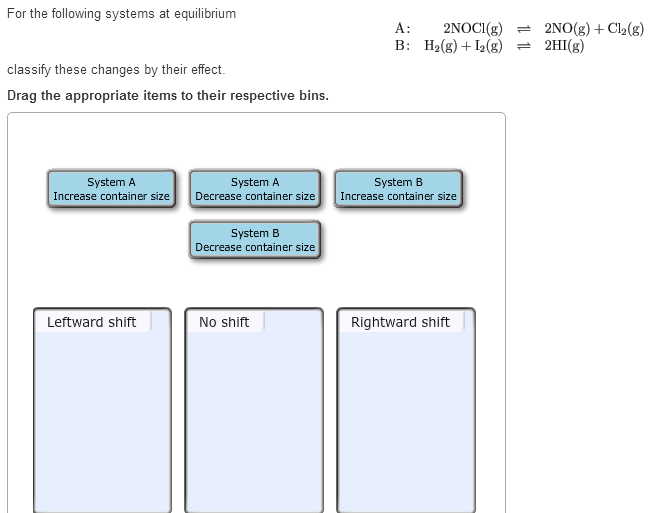
Solved For The Following Systems At Equilibrium A 2nocl G Chegg Consider the number of moles of gaseous reactants and products for system a when determining the direction of the shift in response to a change in container size. To understand how changes in container size affect systems at equilibrium, we can use le chatelier's principle, which states that a system at equilibrium will adjust to counteract changes imposed on it. let's analyze systems a and b: system a: 2nocl(g) ⇌ 2no(g) c l2(g).

Solved Consider The Following Equilibrium Chegg System a: 2nocl (g) ⇌ 2no (g) cl2 (g) in this system, there are 2 moles of gas on both sides of the equation. therefore, changing the container size will not shift the equilibrium. Part a: for system a (2nocl (g) ⇌ 2no (g) cl2 (g)): increasing the container size will decrease the pressure, causing the system to shift towards the side with more moles of gas to counteract the change. At equilibrium, it is given that the moles of no are 0.4. from the stoichiometry of the reaction, for every 2 moles of nocl that dissociate, 2 moles of no and 1 mole of cl2 are produced. Consider the following equilibrium: 2no g cl2 g 2nocl g Δg0=−41.kj now suppose a reaction vessel is filled with 0.258 atm of chlorine cl2 and 8.51 atm of nitrosyl chloride nocl at 508. °c.

Solved For The Following Systems At Equilibrium Chegg At equilibrium, it is given that the moles of no are 0.4. from the stoichiometry of the reaction, for every 2 moles of nocl that dissociate, 2 moles of no and 1 mole of cl2 are produced. Consider the following equilibrium: 2no g cl2 g 2nocl g Δg0=−41.kj now suppose a reaction vessel is filled with 0.258 atm of chlorine cl2 and 8.51 atm of nitrosyl chloride nocl at 508. °c. At a particular temperature the following system is at equilibrium in a closed container: 2nocl (g) 2no (g) cl2(g) analysis of the equilibrium mixture shows it to have the following composition: p nocl = 1.25 atm. p no = 1.75×10 2 atm. p cl2 = 0.390 atm. calculate the value of k p for this system at this temperature. To classify the change in system a when the container size is increased, observe that increasing volume leads the equilibrium to shift towards the side with more moles of gas. #solution : by increasing the volume the equilibrium shifts to the more number of moles direction. Calculate the equilibrium concentration of cl 2 (g). upload your school material for a more relevant answer. the equilibrium concentration of cl2 (g) can be calculated using the given initial amounts of nocl and cl2, the stoichiometry of the balanced equation, and the equilibrium constant (k). For the following systems at equilibrium a:2nocl (g)⇌2no (g) cl2 (g) b: h2 (g) i2 (g)⇌2hi (g) classify these changes by their effect. drag the appropriate items to their respective bins.

Solved Consider The Following Equilibrium Chegg At a particular temperature the following system is at equilibrium in a closed container: 2nocl (g) 2no (g) cl2(g) analysis of the equilibrium mixture shows it to have the following composition: p nocl = 1.25 atm. p no = 1.75×10 2 atm. p cl2 = 0.390 atm. calculate the value of k p for this system at this temperature. To classify the change in system a when the container size is increased, observe that increasing volume leads the equilibrium to shift towards the side with more moles of gas. #solution : by increasing the volume the equilibrium shifts to the more number of moles direction. Calculate the equilibrium concentration of cl 2 (g). upload your school material for a more relevant answer. the equilibrium concentration of cl2 (g) can be calculated using the given initial amounts of nocl and cl2, the stoichiometry of the balanced equation, and the equilibrium constant (k). For the following systems at equilibrium a:2nocl (g)⇌2no (g) cl2 (g) b: h2 (g) i2 (g)⇌2hi (g) classify these changes by their effect. drag the appropriate items to their respective bins.
Comments are closed.