Solved Given A Concentration Of A Solution Determine The Amount Of

Solved Solution Concentration The Concentration Of A Chegg Concentration, i.e., the amount of solute in a given amount of solution, is described along with its different units. preparation of and dilution of the solution are also presented. The concentration calculator is a tool that consists of five methods for calculating concentration in chemistry. for example, you can convert the molarity into percentage concentration (or vice versa) with a known molar mass of the dissolved substance and the density of the solution. in addition, you can calculate the mass of the substance per 100 g of water if the percentage concentration is.

Solved Given A Concentration Of A Solution Determine The Chegg Mass of solute = 34 g therefore, the mass required is 34 g. in chemistry, we are often required to calculate the concentration of the solution. the above mentioned methods of expressing the concentration of a solution are important. the solved examples are helpful for a better understanding of the concept of concentration of a solution. Perfect for students and professionals, this tool helps you determine molarity, molality, and percentage concentration quickly and accurately. ideal for chemistry homework, lab work, and scientific research. Concentration calculation quantifies solute amount within a solvent or mixture precisely. it is essential in chemistry, biology, and engineering. this article explores key formulas, common values, and real world applications of concentration calculations in detail. mastery of these concepts ensures accurate experimental and industrial outcomes. – to calculate molar concentration, we must find both the amount of ethanol and the volume of the solution. – the volume is given as 3.50 l, so all we need to do is convert the mass of ethanol to the corresponding amount of ethanol in moles:.
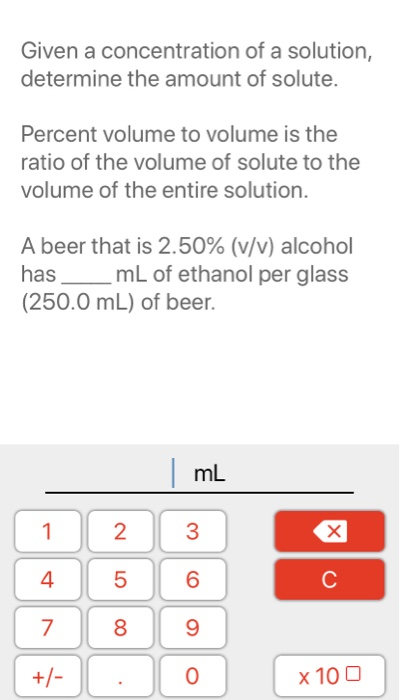
Solved Given A Concentration Of A Solution Determine The Chegg Concentration calculation quantifies solute amount within a solvent or mixture precisely. it is essential in chemistry, biology, and engineering. this article explores key formulas, common values, and real world applications of concentration calculations in detail. mastery of these concepts ensures accurate experimental and industrial outcomes. – to calculate molar concentration, we must find both the amount of ethanol and the volume of the solution. – the volume is given as 3.50 l, so all we need to do is convert the mass of ethanol to the corresponding amount of ethanol in moles:. Calculate solution concentrations from solute and solvent amounts. convert between molarity, molality, mass percentage, and other concentration units with step by step solutions. Concentration quantifies the amount of a specific substance, known as the solute, present within a given amount of a mixture, typically a solution. this measurement indicates how “strong” or “weak” a solution is, influencing its behavior in chemical reactions and its utility in diverse applications. Learn how to calculate the concentration of a solution using mass and volume. includes worked examples, units, and conversions between cm³ and dm³. There are several ways to express the concentration of a solution: let's dive into each of these methods to understand how they are calculated. molarity is one of the most common ways to express concentration. it is defined as the number of moles of solute per liter of solution. $$m = \frac {n} {v}$$m = v n. where:.

Solved Given A Concentration Of A Solution Determine The Chegg Calculate solution concentrations from solute and solvent amounts. convert between molarity, molality, mass percentage, and other concentration units with step by step solutions. Concentration quantifies the amount of a specific substance, known as the solute, present within a given amount of a mixture, typically a solution. this measurement indicates how “strong” or “weak” a solution is, influencing its behavior in chemical reactions and its utility in diverse applications. Learn how to calculate the concentration of a solution using mass and volume. includes worked examples, units, and conversions between cm³ and dm³. There are several ways to express the concentration of a solution: let's dive into each of these methods to understand how they are calculated. molarity is one of the most common ways to express concentration. it is defined as the number of moles of solute per liter of solution. $$m = \frac {n} {v}$$m = v n. where:.

Solved 5 Given A Concentration Of A Solution Determine The Chegg Learn how to calculate the concentration of a solution using mass and volume. includes worked examples, units, and conversions between cm³ and dm³. There are several ways to express the concentration of a solution: let's dive into each of these methods to understand how they are calculated. molarity is one of the most common ways to express concentration. it is defined as the number of moles of solute per liter of solution. $$m = \frac {n} {v}$$m = v n. where:.
Comments are closed.