
Solved How Do Ionic Bonds Differ From Covalent Chegg How do ionic bonds differ from covalent bonds? electrons are not shared between two atomic nuclei in ionic bonds as they are in covalent bonds. ionic bonds are not important in biological systems, whereas covalent bonds are. How do ionic bonds differ from covalent bonds? electrons are not shared between two atomic nuclei in ionic bonds as they are in covalent bonds. what determines whether an atom will form a chemical bond with another atom?.

Solved Covalent Bonds Differ From Ionic Bonds In That A Chegg How do ionic bonds differ from covalent bonds a. ionic bonds are not important in biological systems, whereas covalent bonds are b. the strength of attraction holding atomic nuclei in an ionic bond is much stronger than in covalent bonds c. ionic bonds cannot easily be broken when exposed to water, whereas covalent bonds are readily broken in. Ionic bonds are attached to positive and negative ions, covalent bongs share pairs of electrons. how does an ionic compound differ from a molecular compound? ionic compounds have a high melting point and it is conductive when dissolved in water, molecular have low melting points and it does not conduct. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. here is an explanation of the difference between ionic and covalent bonds, examples of each bond type, and a look at how to tell which type of bond will form. Ionic bonds and covalent bonds are two fundamental types of chemical bonds that connect atoms in molecules, and they differ significantly in how they involve electrons. ionic bonds: in an ionic bond, electrons are transferred from one atom to another.

Distinguishing Between Ionic And Covalent Compounds Lab Pdf The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. here is an explanation of the difference between ionic and covalent bonds, examples of each bond type, and a look at how to tell which type of bond will form. Ionic bonds and covalent bonds are two fundamental types of chemical bonds that connect atoms in molecules, and they differ significantly in how they involve electrons. ionic bonds: in an ionic bond, electrons are transferred from one atom to another. Electrons are not shared between two atomic i nuclei in ionic bonds as they are in covalent bonds. the strength of attraction holding atomic nuclei in an ionic bond is much stronger than in covalent bonds. ionic bonds are not important in biological systems, whereas covalent bonds are. Enhanced with ai, our expert help has broken down your problem into an easy to learn solution you can count on. here’s the best way to solve it. ionic bonds and covalent bonds are two types of ch not the question you’re looking for? post any question and get expert help quickly. Ionic bonds and covalent bonds are two types of chemical bonds that hold atoms together, but they do so in different ways: ionic bonds: formation: ionic bonds form when one atom donates an electron to another atom. How do ionic bonds differ from covalent bonds? ionic bonds are not important in biological systems, whereas covalent bonds are. the strength of attraction holding atomic nuclei in an ionic bond is much stronger than in covalent bonds.
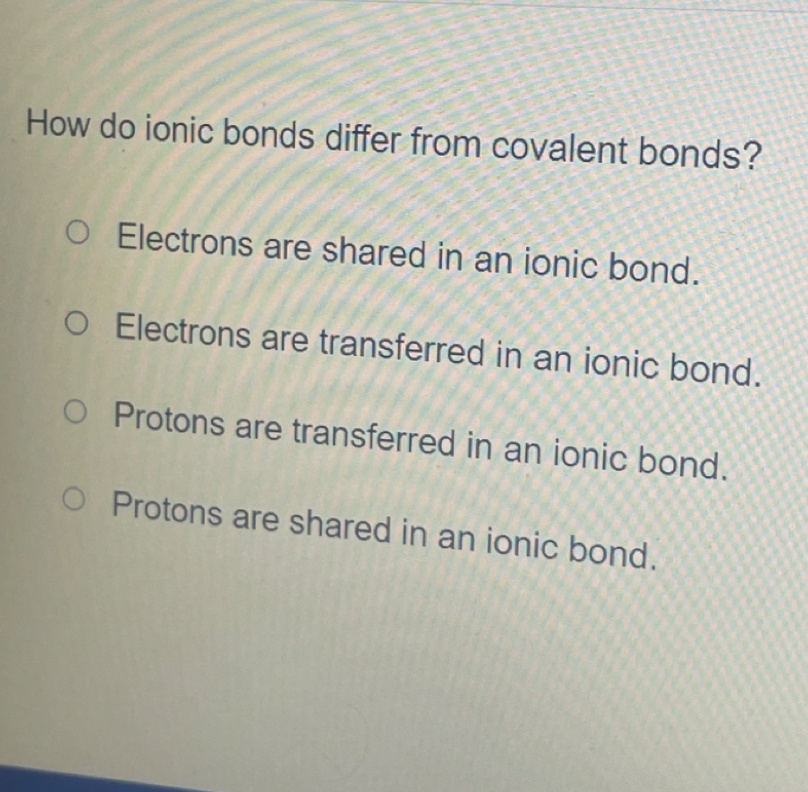
Solved How Do Ionic Bonds Differ From Covalent Chegg Electrons are not shared between two atomic i nuclei in ionic bonds as they are in covalent bonds. the strength of attraction holding atomic nuclei in an ionic bond is much stronger than in covalent bonds. ionic bonds are not important in biological systems, whereas covalent bonds are. Enhanced with ai, our expert help has broken down your problem into an easy to learn solution you can count on. here’s the best way to solve it. ionic bonds and covalent bonds are two types of ch not the question you’re looking for? post any question and get expert help quickly. Ionic bonds and covalent bonds are two types of chemical bonds that hold atoms together, but they do so in different ways: ionic bonds: formation: ionic bonds form when one atom donates an electron to another atom. How do ionic bonds differ from covalent bonds? ionic bonds are not important in biological systems, whereas covalent bonds are. the strength of attraction holding atomic nuclei in an ionic bond is much stronger than in covalent bonds.
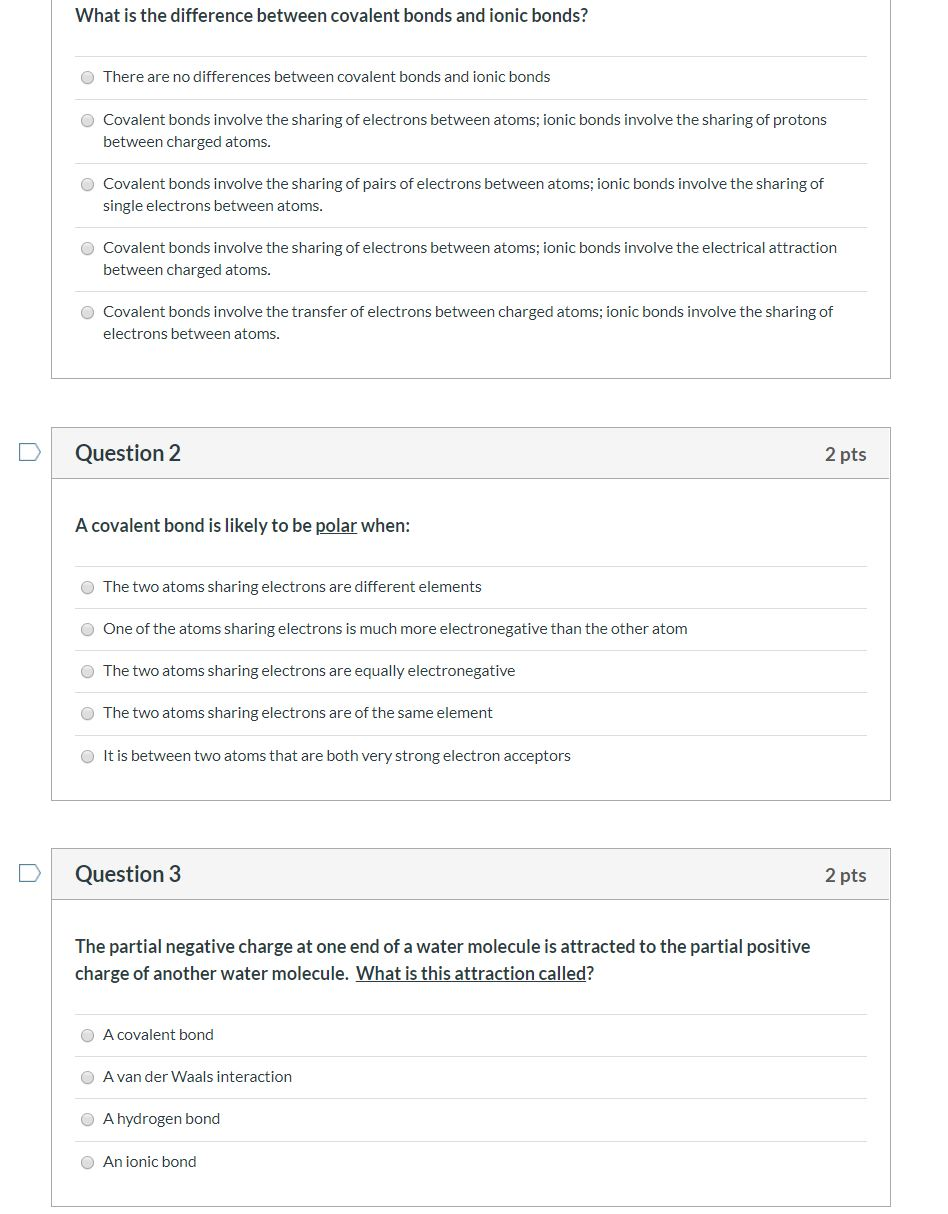
Solved What Is The Difference Between Covalent Bonds And Chegg Ionic bonds and covalent bonds are two types of chemical bonds that hold atoms together, but they do so in different ways: ionic bonds: formation: ionic bonds form when one atom donates an electron to another atom. How do ionic bonds differ from covalent bonds? ionic bonds are not important in biological systems, whereas covalent bonds are. the strength of attraction holding atomic nuclei in an ionic bond is much stronger than in covalent bonds.

Which Statement Comparing Ionic Bonds And Covalent Chegg
