Solved If A Voltaic Cell Is Constructed Consisting Of A Chegg
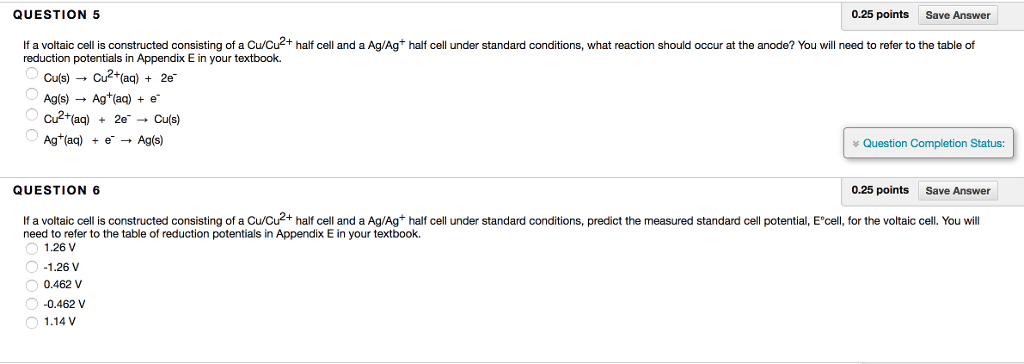
Solved If A Voltaic Cell Is Constructed Consisting Of A Chegg Question: if a voltaic cell is constructed consisting of a cu cu^2 half cell and a ag ag^ half cell under standard conditions, what reaction should occur at the anode? you will need to refer to the table of reduction potentials in appendix e in your textbook. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. it consists of two separate half cells.

Solved If A Voltaic Cell Is Constructed Consisting Of A Chegg If a voltaic cell is constructed consisting of a cu cu2 half cell and a ag ag half cell under standard conditions, predict the measured standard cell potential, e°cell, for the voltaic cell. you will need to refer to the table of reduction potentials in appendix e in your textbook. Electrochemical cells, such as voltaic cells, convert chemical energy into electrical energy through redox reactions. in a voltaic cell, oxidation occurs at the anode and reduction at the cathode, allowing for the flow of electrons through an external circuit. Calculate the standard cell potential produced by a voltaic cell consisting of a gold electrode in contact with a solution of au3 ions and a silver electrode in contact with a solution of ag ions. Calculate the standard cell potential produced by a voltaic cell consisting of a gold electrode in contact with a solution of au3 ions and a silver electrode in contact with a solution of ag ions.
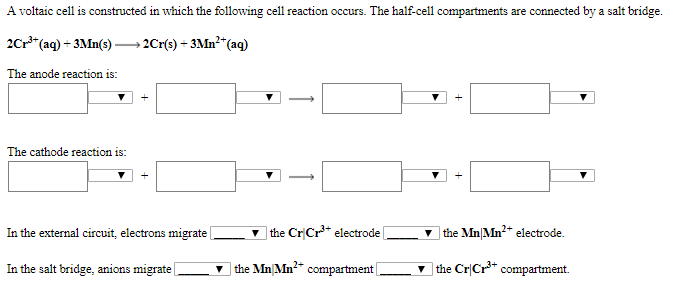
Solved A Voltaic Cell Is Constructed In Which The Following Chegg Calculate the standard cell potential produced by a voltaic cell consisting of a gold electrode in contact with a solution of au3 ions and a silver electrode in contact with a solution of ag ions. Calculate the standard cell potential produced by a voltaic cell consisting of a gold electrode in contact with a solution of au3 ions and a silver electrode in contact with a solution of ag ions. When we consider a voltaic cell, there are two electrodes involved: the cathode and the anode, each with differing potentials. the cathode has a higher electrode potential, meaning it attracts electrons. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. To determine how the observed potentials differ from the expected values, the % relative errors are used. these % relative errors varied from as little as 5% to as large as 60%. name one factor that might account for these differences. In this experiment, you will prepare a variety of semi microscale voltaic cells in a 24 well test plate. a voltaic cell is constructed by using two metal electrodes and solutions of their respective salts (the electrolyte component of the cell) with known molar concentrations.
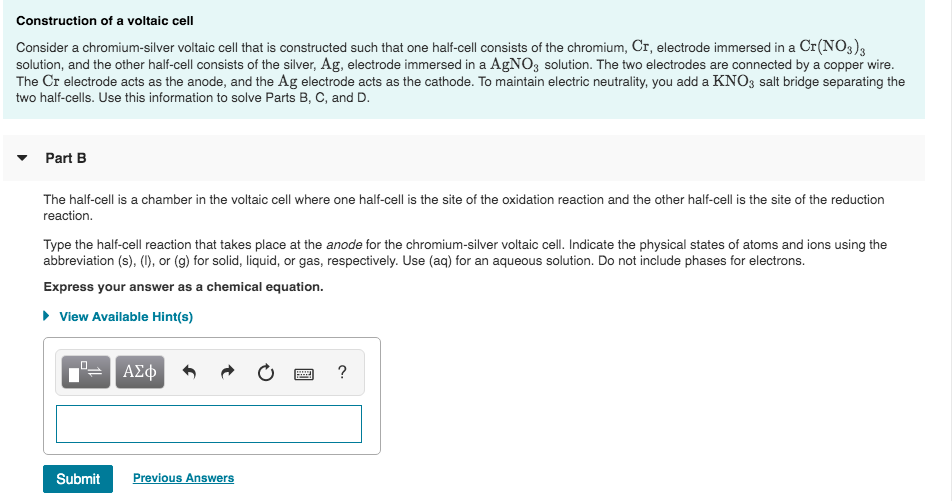
Solved Construction Of A Voltaic Cell Consider A Chegg When we consider a voltaic cell, there are two electrodes involved: the cathode and the anode, each with differing potentials. the cathode has a higher electrode potential, meaning it attracts electrons. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. To determine how the observed potentials differ from the expected values, the % relative errors are used. these % relative errors varied from as little as 5% to as large as 60%. name one factor that might account for these differences. In this experiment, you will prepare a variety of semi microscale voltaic cells in a 24 well test plate. a voltaic cell is constructed by using two metal electrodes and solutions of their respective salts (the electrolyte component of the cell) with known molar concentrations.
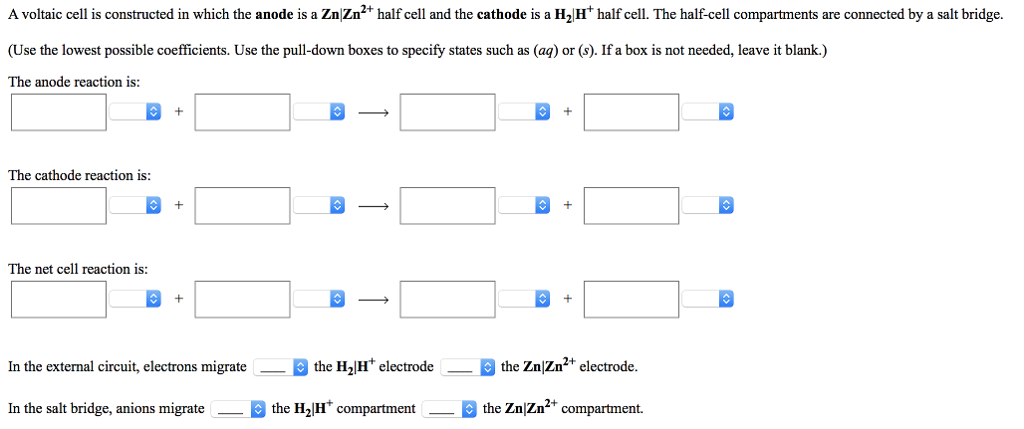
Solved A Voltaic Cell Is Constructed In Which The Following Chegg To determine how the observed potentials differ from the expected values, the % relative errors are used. these % relative errors varied from as little as 5% to as large as 60%. name one factor that might account for these differences. In this experiment, you will prepare a variety of semi microscale voltaic cells in a 24 well test plate. a voltaic cell is constructed by using two metal electrodes and solutions of their respective salts (the electrolyte component of the cell) with known molar concentrations.

Solved A Voltaic Cell Is Constructed In Which The Following Chegg
Comments are closed.