
Lab 4 Investigating Ionic And Covalent Compounds Pdf Chemical Compound br₂ h₂o ca(cio) bf3 al(oh), hf pfs ti(so4)2 ionic and covalent bonding page 8 of 8 show transcribed image text there are 2 steps to solve this one. Our expert help has broken down your problem into an easy to learn solution you can count on. determine whether each of the following is a covalent or ionic compound drag the appropriate compounds to their respective bins. there are 2 steps to solve this one.

Solved Ionic Or Covalent Determine If The Compounds Are Chegg Classify the following compounds as having covalent or ionic bonds. drag the appropriate compounds to their respective bins. covalent bonds: carbon tetrachloride dinitrogen monoxide ionic bonds: rubidium oxide aluminum carbide magnesium chloride barium fluoride. Therefore, ch₄ is a covalent bond. analyze the bond in mgo (magnesium oxide). magnesium (mg) is a metal and oxygen (o) is a nonmetal. the electronegativity difference is significant, leading to the transfer of electrons from mg to o, forming mg² and o² ions. hence, mgo is an ionic bond. Classify the following compounds as ionic or covalent: kcl, crcl₃, cl₂o. ionic bonds occur between a metal and a nonmetal. covalent bonds occur between two nonmetals. what is the formula for an ionic compound containing ba²⁺ ions and cl⁻ ions? what type of bonding is present in c₁₂h₂₂o₁₁? c₁₂h₂₂o₁₁ is a covalent compound. Determine if the compounds are ionic or covalent, and then whether polar or nonpolar. \begin {tabular} {|l|l|l|} \hline compound & lonic or covalent & if covalent, polar or nonpolar? \\ \hline br2 & & \\ \hline h2o & & \\ \hline ca (clo)2 & & \\ \hline bf & & \\ \hline al (oh)2 & & \\ \hline hf & & \\ \hline pf & & \\ \hline ti (so3)2 & & \\.

Solved Ionic Versus Covalent Bonding Can You Determine If Chegg Classify the following compounds as ionic or covalent: kcl, crcl₃, cl₂o. ionic bonds occur between a metal and a nonmetal. covalent bonds occur between two nonmetals. what is the formula for an ionic compound containing ba²⁺ ions and cl⁻ ions? what type of bonding is present in c₁₂h₂₂o₁₁? c₁₂h₂₂o₁₁ is a covalent compound. Determine if the compounds are ionic or covalent, and then whether polar or nonpolar. \begin {tabular} {|l|l|l|} \hline compound & lonic or covalent & if covalent, polar or nonpolar? \\ \hline br2 & & \\ \hline h2o & & \\ \hline ca (clo)2 & & \\ \hline bf & & \\ \hline al (oh)2 & & \\ \hline hf & & \\ \hline pf & & \\ \hline ti (so3)2 & & \\. Sure! let's go through each compound and determine whether it's ionic or covalent, and then name it appropriately. na₂co₃ (sodium carbonate): type: ionic. this compound consists of a metal (sodium) and a polyatomic ion (carbonate). name: sodium carbonate is the correct name for this ionic compound. p₂o₅: type: covalent. this compound is. Using the periodic table, predict whether the following chlorides are ionic or covalent: sicl 4, pcl 3, cacl 2, cscl, cucl 2, and crcl 3. for each of the following compounds, state whether it is ionic or covalent. if it is ionic, write the symbols for the ions involved:. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. here is an explanation of the difference between ionic and covalent bonds, examples of each bond type, and a look at how to tell which type of bond will form. Step 1: identify the electronegativity of the given elements. step 2: calculate the difference between the electronegativities Δ e n of the given elements. if Δ e n <0.4, then it's a pure.
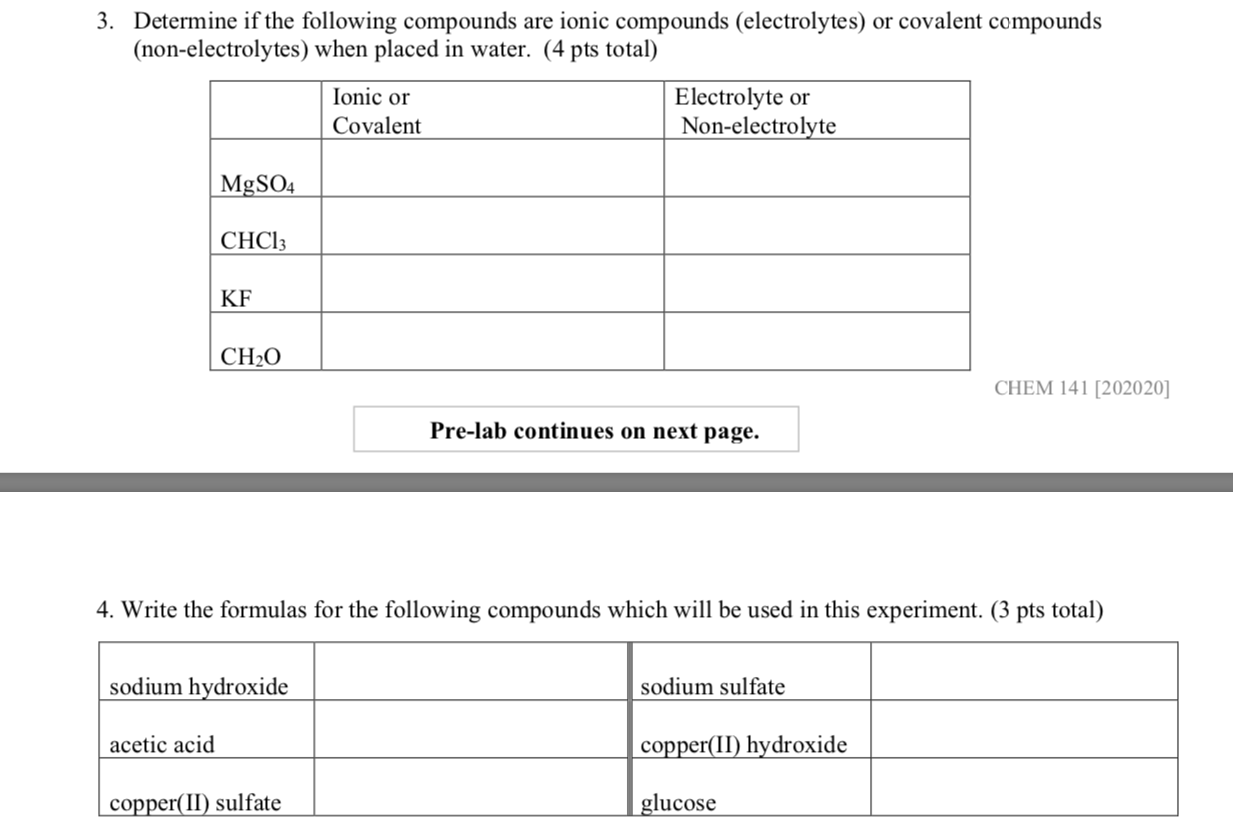
Solved 3 Determine If The Following Compounds Are Ionic Chegg Sure! let's go through each compound and determine whether it's ionic or covalent, and then name it appropriately. na₂co₃ (sodium carbonate): type: ionic. this compound consists of a metal (sodium) and a polyatomic ion (carbonate). name: sodium carbonate is the correct name for this ionic compound. p₂o₅: type: covalent. this compound is. Using the periodic table, predict whether the following chlorides are ionic or covalent: sicl 4, pcl 3, cacl 2, cscl, cucl 2, and crcl 3. for each of the following compounds, state whether it is ionic or covalent. if it is ionic, write the symbols for the ions involved:. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. here is an explanation of the difference between ionic and covalent bonds, examples of each bond type, and a look at how to tell which type of bond will form. Step 1: identify the electronegativity of the given elements. step 2: calculate the difference between the electronegativities Δ e n of the given elements. if Δ e n <0.4, then it's a pure.
