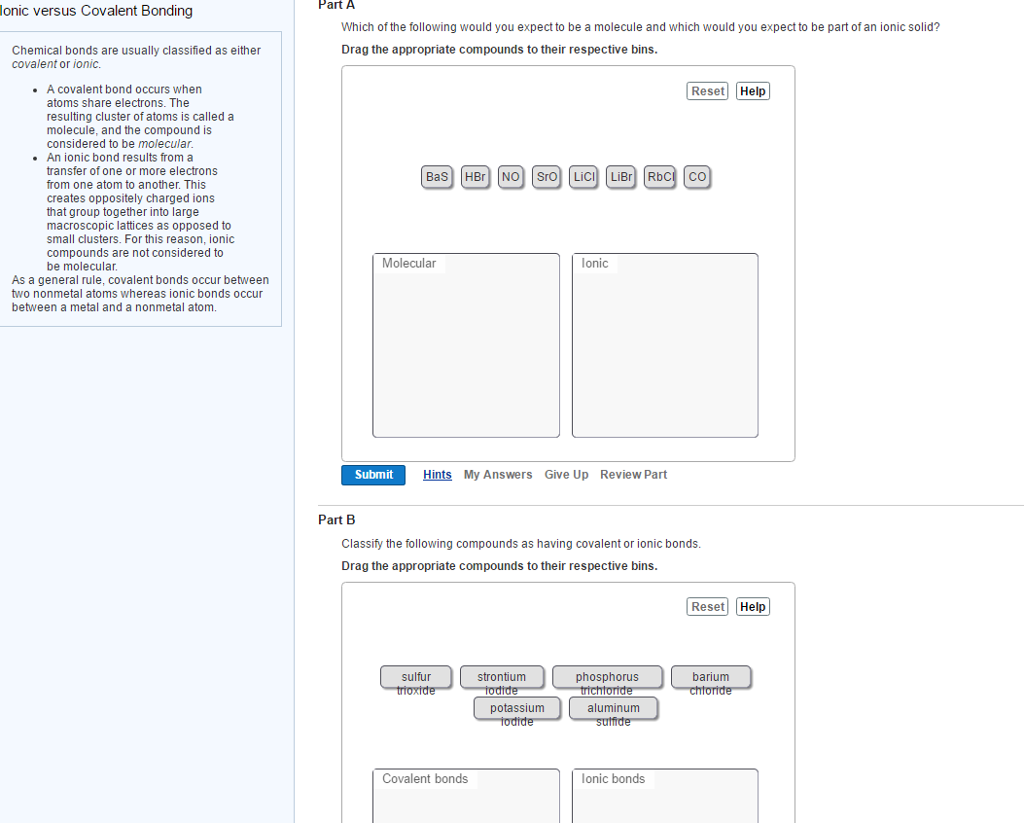
Solved Ionic Versus Covalent Bonding Chemical Bonds Are Chegg Ionic versus covalent bonding chemical bonds are usually classified as either covalent or ionic. a covalent bond occurs when atoms share electrons. the resulting cluster of atoms is called a molecule, and the compound is considered to be molecular. an ionic bond results from a transfer of one or more electrons from one atom to another. Our expert help has broken down your problem into an easy to learn solution you can count on. there are 2 steps to solve this one. ionic and covalent bonds are two different types of chemical bonds that form between atoms to create not the question you’re looking for? post any question and get expert help quickly.

Solved Ionic Versus Covalent Bonding Can You Determine If Chegg What is the difference between an ionic bond and a covalent bond? give an example of a compound formed by each type of bond. 2. what is the ph scale, and how does it relate to acidity and alkalinity? give an example of a substance with a ph value and explain whether it is acidic, neutral, or alkaline. 3. In an ionic bond, an electron is donated. in a covalent bond, the electron is shared. ionic and covalent bonds are the two main types of chemical bonding. a chemical bond is a link formed between two or more atoms or ions. the main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Ionic and covalent bonds are chemical bonds. what specific property of metals accounts for their unusual electrical conductivity? the freedom of electrons to move in a network of metal atoms accounts for high electrical conductivity. what properties of metals contribute to their tendency to form metallic bonds?. Ionic and covalent bonds are the two extremes of bonding. polar covalent is the intermediate type of bonding between the two extremes. some ionic bonds contain covalent characteristics and some covalent bonds are partially ionic. for example, most carbon based compounds are covalently bonded but can also be partially ionic.
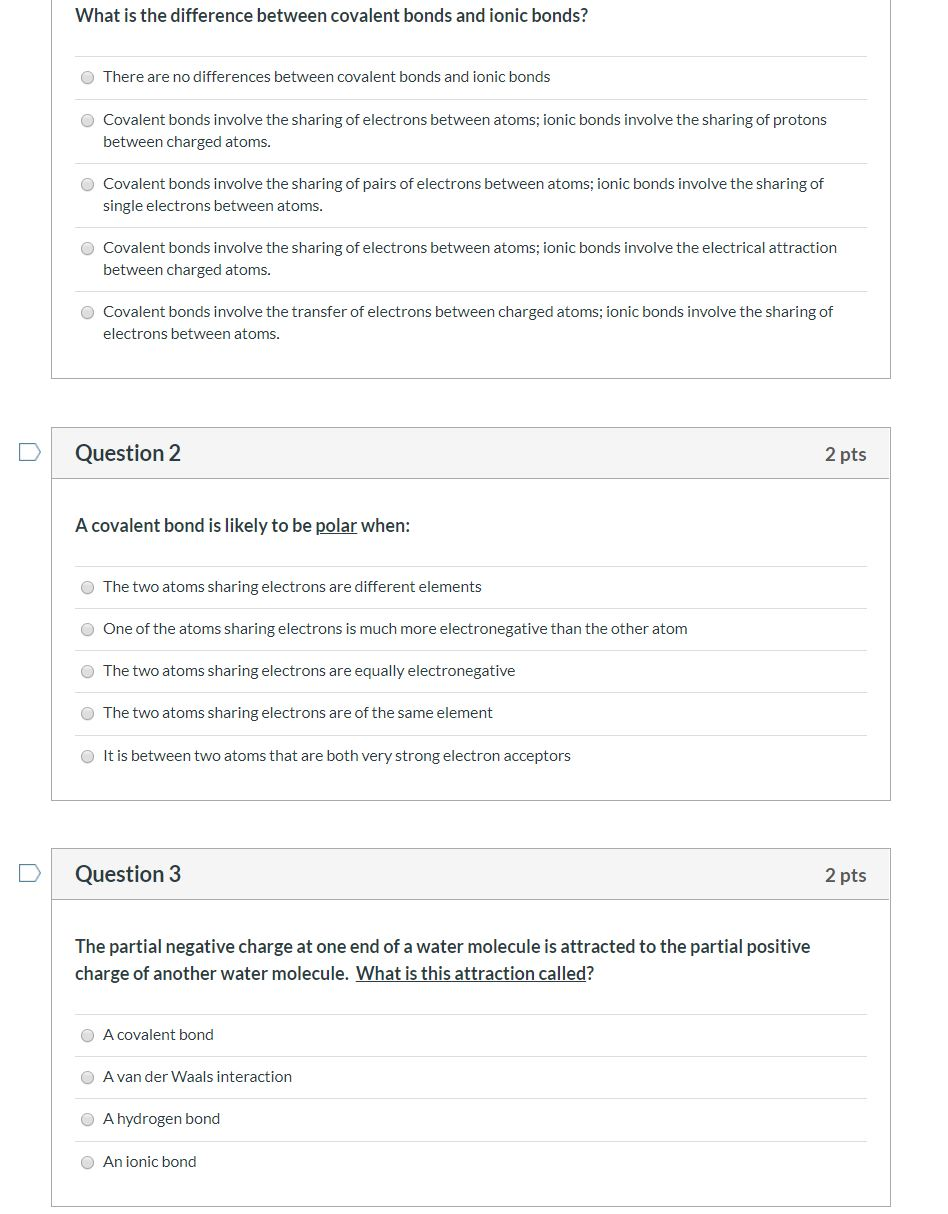
Solved What Is The Difference Between Covalent Bonds And Chegg Ionic and covalent bonds are chemical bonds. what specific property of metals accounts for their unusual electrical conductivity? the freedom of electrons to move in a network of metal atoms accounts for high electrical conductivity. what properties of metals contribute to their tendency to form metallic bonds?. Ionic and covalent bonds are the two extremes of bonding. polar covalent is the intermediate type of bonding between the two extremes. some ionic bonds contain covalent characteristics and some covalent bonds are partially ionic. for example, most carbon based compounds are covalently bonded but can also be partially ionic. Study with quizlet and memorize flashcards containing terms like what is the main distinction between ionic and covalent bonding, how is electronegativity used in determining the ionic or covalent character or the bonding between two elements, chemical bonds and more. Here's a quick summary of the differences between ionic and covalent bonds, their properties, and how to recognize them: bond between metal and nonmetal. the nonmetal attracts the electron, so it's like the metal donates its electron to it. bond between two nonmetals with similar electronegativities. atoms share electrons in their outer orbitals. Covalent bonding is a form of chemical bonding between two non metallic atoms which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds. ionic bond, also known as electrovalent bond, is a type of bond formed from the electrostatic attraction between oppositely charged ions in a chemical compound . A covalent bond is a type of chemical bonding resulting from the mutual sharing of electrons between two atoms of the same or different elements. the ionic bond is an electrostatic interaction between two oppositely charged atoms or ions with different electronegativities as a result of the transfer of electrons from one chemical species to.

Solved Covalent Bonds Differ From Ionic Bonds In That A Chegg Study with quizlet and memorize flashcards containing terms like what is the main distinction between ionic and covalent bonding, how is electronegativity used in determining the ionic or covalent character or the bonding between two elements, chemical bonds and more. Here's a quick summary of the differences between ionic and covalent bonds, their properties, and how to recognize them: bond between metal and nonmetal. the nonmetal attracts the electron, so it's like the metal donates its electron to it. bond between two nonmetals with similar electronegativities. atoms share electrons in their outer orbitals. Covalent bonding is a form of chemical bonding between two non metallic atoms which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds. ionic bond, also known as electrovalent bond, is a type of bond formed from the electrostatic attraction between oppositely charged ions in a chemical compound . A covalent bond is a type of chemical bonding resulting from the mutual sharing of electrons between two atoms of the same or different elements. the ionic bond is an electrostatic interaction between two oppositely charged atoms or ions with different electronegativities as a result of the transfer of electrons from one chemical species to.
