Solved Part A Indicate Whether Each Of The Following Pairs Chegg
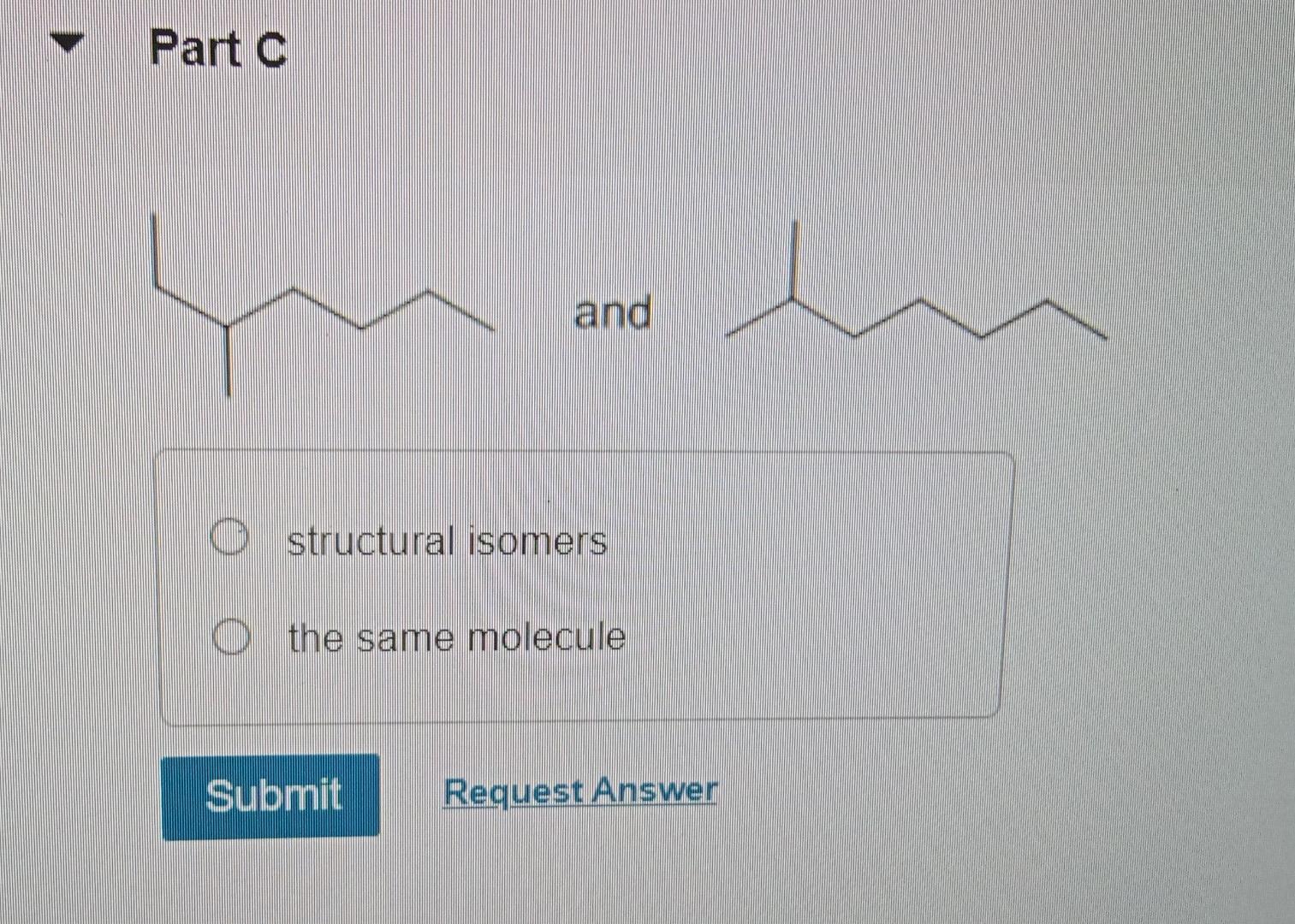
Solved Indicate Whether Each Of The Following Pairs Of Part Chegg Part a indicate whether each of the following pairs represents structural isomers, cis trans isomers, or identical compounds. drag the appropriate items to their respective bins. We have an expert written solution to this problem! complete and balance the equation for the reaction of sulfuric acid and aluminum hydroxide. if 29.7 ml of 0.205 m koh is required to completely neutralize 25.0 ml of a ch3cooh solution, what is the molarity of the acetic acid solution? 0.244 m.

Solved Question 2 For Each Of The Following Pairs Of Chegg First, we need to understand the problem by identifying the chemical structure of each compound using smiles notation. list each given pair of smiles strings and convert them to their respective chemical structures. Indicate whether or not the two members of each of the following pairs of substances constitute a conjugate acid base pair: a) nh3 and nh^2− [ select ] ["yes, these are a conjugate acid base pair", "no, these are not a conjugate acid base pair"]. Give the relationships between the following pairs of structures. the possible relationships are as follows: same compound, cis trans isomers, constitutional (structural) isomers, and not isomers (different molecular formula). Enantiomers are chiral molecules that are non superimposable mirror images of each other by any combination of rotations, translations, and some conformational changes. in this example, we have two molecules that are mirror images of each other, which means that they are enantiomers.

Solved Indicate Whether Or Not The Following Pairs Of Chegg Give the relationships between the following pairs of structures. the possible relationships are as follows: same compound, cis trans isomers, constitutional (structural) isomers, and not isomers (different molecular formula). Enantiomers are chiral molecules that are non superimposable mirror images of each other by any combination of rotations, translations, and some conformational changes. in this example, we have two molecules that are mirror images of each other, which means that they are enantiomers. Part a indicate whether each of the following pairs of formulas represents structural isomers or the same molecule. drag the appropriate items to their respective bins. Find step by step chemistry solutions and the answer to the textbook question for each pair of the following molecules, indicate whether its members are identical, structural isomers, conformers, or stereoisomers. On the basis of their electron configurations, predict the formula of the simple binary ionic compound likely to form when the following pairs of elements react with each other. Before identifying the relationships, let's define the possible pair types: anomers are isomers differing at the anomeric carbon (c 1 in aldoses). epimers are isomers differing at one of several stereocenters.
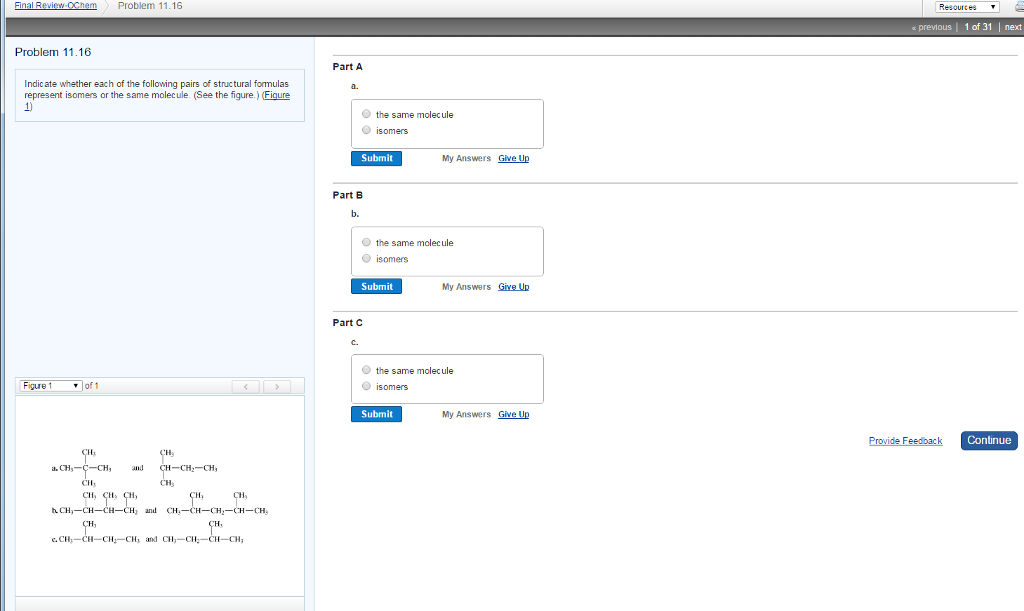
Solved Indicate Whether Each Of The Following Pairs Of Chegg Part a indicate whether each of the following pairs of formulas represents structural isomers or the same molecule. drag the appropriate items to their respective bins. Find step by step chemistry solutions and the answer to the textbook question for each pair of the following molecules, indicate whether its members are identical, structural isomers, conformers, or stereoisomers. On the basis of their electron configurations, predict the formula of the simple binary ionic compound likely to form when the following pairs of elements react with each other. Before identifying the relationships, let's define the possible pair types: anomers are isomers differing at the anomeric carbon (c 1 in aldoses). epimers are isomers differing at one of several stereocenters.
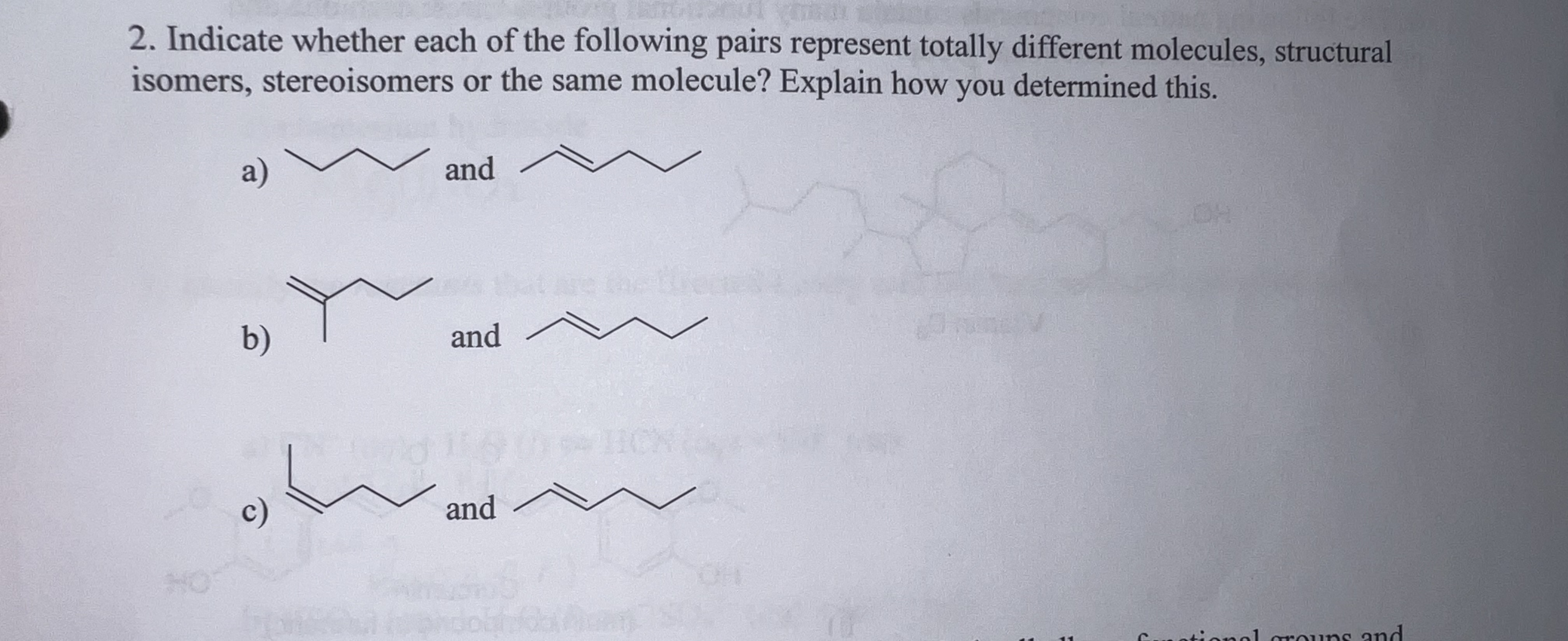
Solved 2 Indicate Whether Each Of The Following Pairs Chegg On the basis of their electron configurations, predict the formula of the simple binary ionic compound likely to form when the following pairs of elements react with each other. Before identifying the relationships, let's define the possible pair types: anomers are isomers differing at the anomeric carbon (c 1 in aldoses). epimers are isomers differing at one of several stereocenters.
Comments are closed.