Solved Part B Given The Two Reactions 3 Pbcl Aq Pb Aq Chegg
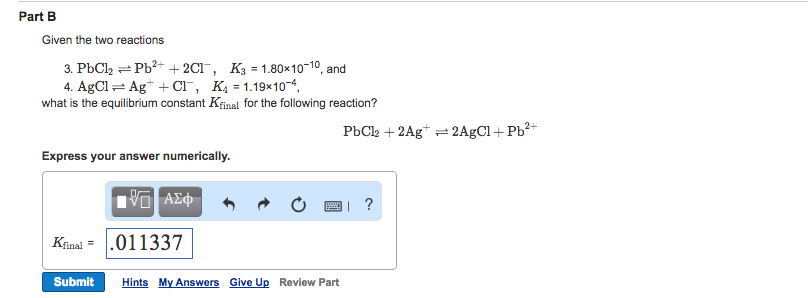
Solved Part B Given The Two Reactions 3 Pbcl Pb2 2cl K3 Chegg Pbcl2(aq)⇌pb2 (aq) 2cl−(aq),k 3 = 1.74×10−10, and 4. agcl(aq)⇌ag (aq) cl−(aq), k 4 =1.24×10−4. what is the equilibrium constant k final for the following reaction?. The first reaction is given as pbcl2 (s)⇌pb2 (aq) 2cl− (aq) with k3 as 1.77×10−10, and the second is agcl (s)⇌ag (aq) cl− (aq) with k4 as 1.12×10−4. to obtain the desired reaction, the first equation can be left unaltered, but the second equation must be reversed and multiplied by two.
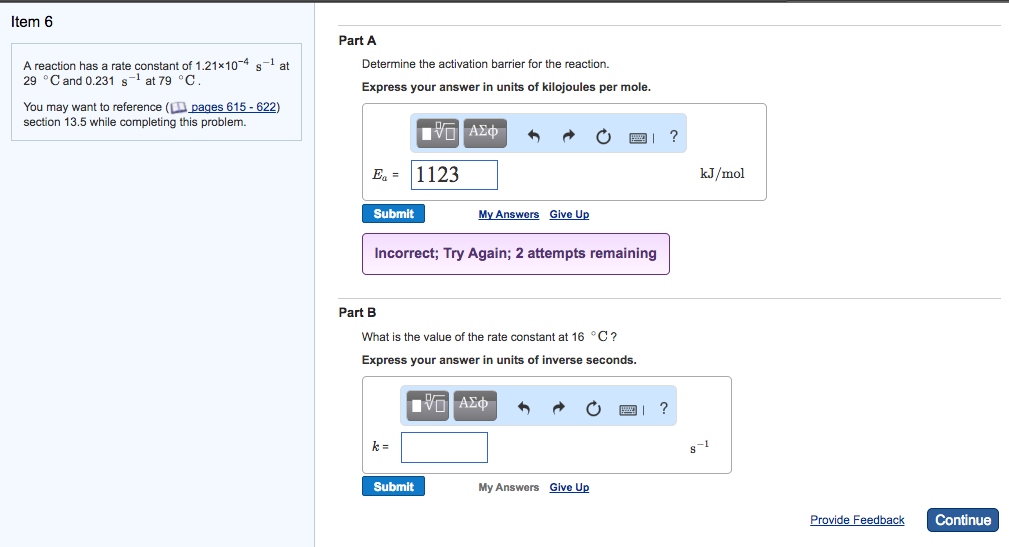
Solved Part B Given The Two Reactions 3 Pbcl Pb2 2cl K3 Chegg Given the two reactions pbcl2 (aq)⇌pb2 (aq) 2cl− (aq), k3 = 1.83×10−10, and agcl (aq)⇌ag (aq) cl− (aq), k4 = 1.22×10−4, what is the equilibrium constant kfinal for the following reaction?. To find the equilibrium constant kfinal for the given reaction, we need to manipulate the given reactions to match the final reaction and then multiply their equilibrium constants. So in this question, you are given with the reaction, which is pbcl2 is associating into pb2 plus ions and 2cl negative ions. okay. and then you have the k3 value, which is the rate constant value, right?. Eso for rk s p a p b seal to the reaction is pb seal to an equilibrium with p b plus two in to see l minus, um, and for the formation of our complex, i honor p b 00 h.
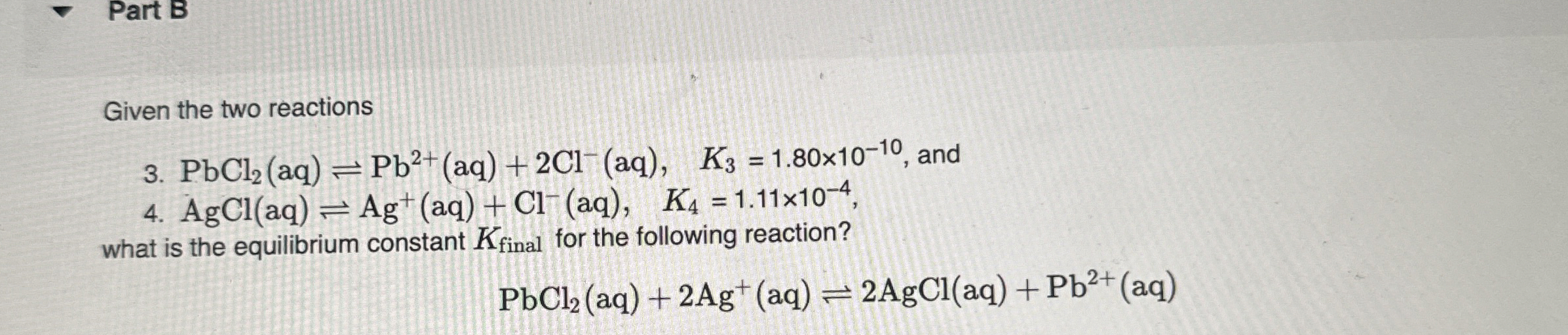
Solved Part B Given The Two Reactions 3 Pbcl 2 Aq Pb So in this question, you are given with the reaction, which is pbcl2 is associating into pb2 plus ions and 2cl negative ions. okay. and then you have the k3 value, which is the rate constant value, right?. Eso for rk s p a p b seal to the reaction is pb seal to an equilibrium with p b plus two in to see l minus, um, and for the formation of our complex, i honor p b 00 h. [solved] given the two reactions 3. pbcl2 (aq) ⇌ pb^2 (aq) 2 cl^ (aq), k3 = 1.77 × 10^ 10, and 4. agcl (aq) ⇌ ag (aq) cl^ (aq), k4 = 1.2. Given the two reactions 3pbcl2(aq)=pb21(aq) 2cl−(aq),k 3 =183×10−10, and 4. agcl(aq)=ag (aq) cl−(aq), k s = 1.15×10−4. what is the equifibrium constant k final for the following reaction?. A more complex problem: the common ion effect calculating the solubility of pb (io 3) 2 in deionized water is a straightforward problem because the solid’s dissolution is the only source of pb 2 and \ (\text {io} 3^ \). but what if we add pb (io 3) 2 to a solution of 0.10 m pb (no 3) 2? before we set‐up and solve this problem algebraically, think about the system’s chemistry and decide. We can use the equilibrium constants, k3 and k4, for the individual reactions given. the reaction equation involves two separate reactions: now, let's combine these two reactions to obtain the desired reaction: pbcl2 (s) 2ag (aq) ⇌ 2agcl (s) pb^2 (aq).
Comments are closed.