Solved Phase Diagram Can Any One Suggest Me The Textbook Chegg
Solved Phase Diagram Can Any One Suggest Me The Textbook Chegg Your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. see answer. We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. for example, a pressure of 50 kpa and a temperature of −10 °c correspond to the region of the diagram labeled “ice.”.

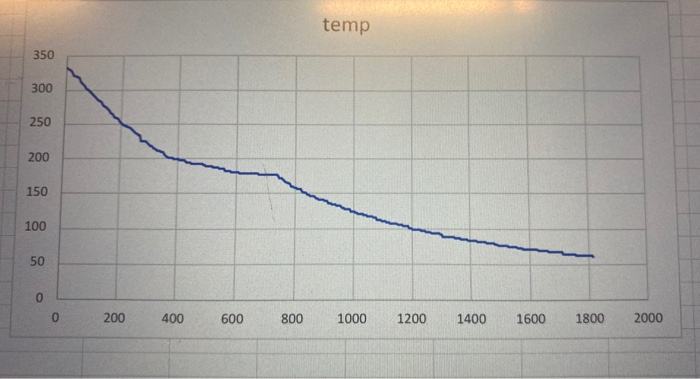
Solved Phase Diagram Chegg Although phase diagrams provide information about systems at equilibrium, they can also assist in predicting phase relations, compositional changes and structures in systems not at equilibrium. Using figure 8.13, calculate the average composition of this alloy. the eutectic and eutectoid reactions are similar in that they both involve the decomposition of a single phase into two solid phases. the –oid suffix indicates that a solid, rather than liquid, phase is decomposing. Phase diagram lab in this lab you will use phase diagrams, tie lines, and the lever rule to determine what phases are present, the composition of those phases (percent component a or b), and the amount of each phase present (percent liquid or solid). Solution (a) we are asked to determine how much sugar will dissolve in 1000 g of water at 90°c. from the solubility limit curve in figure 9.1, at 90° c the maximum concentration of sugar in the syrup is about 77 wt%.
Solved Phase Diagram Chegg Phase diagram lab in this lab you will use phase diagrams, tie lines, and the lever rule to determine what phases are present, the composition of those phases (percent component a or b), and the amount of each phase present (percent liquid or solid). Solution (a) we are asked to determine how much sugar will dissolve in 1000 g of water at 90°c. from the solubility limit curve in figure 9.1, at 90° c the maximum concentration of sugar in the syrup is about 77 wt%. Each problem printed in the text is reproduced in this manual, followed by a worked out solution. if a figure or table accompanies a problem in the text, it is also reproduced here. included within a solution may be an additional figure or table that does not appear in the text. The states of matter exhibited by a substance under different temperatures and pressures can be summarized graphically in a phase diagram, which is a plot of pressure versus temperature. We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. for example, a pressure of 50 kpa and a temperature of −10 °c correspond to the region of the diagram labeled “ice.”. How can phase diagrams be used? phase diagrams can be used to determine the conditions under which substances will undergo a change of state (change of phase). for example, a phase diagram for water will tell you which state water will be in at a given temperature and pressure.
Comments are closed.