Solved Question 11 The Reaction Pb No3 2 Aq 2kl Aq Chegg
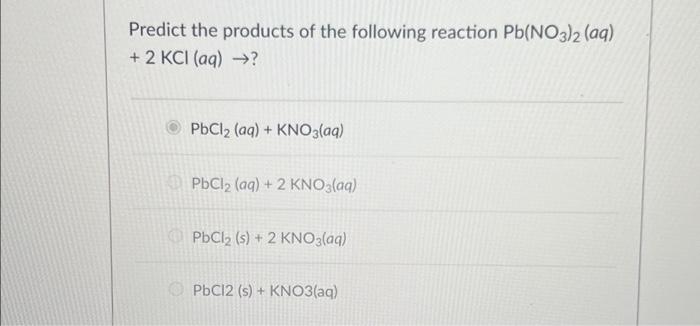
Solved Predict The Products Of The Following Reaction Chegg This problem has been solved! you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Suppose two chemical reactions are linked together in a way that the o2 produced in the first reaction goes on to react completely with mg to form mgo in the second reaction.

Answered Place Each Reactions In The Correct Category A Pb No3 2 Aq H P b2 (aq) 2i −(aq) → p bi 2 (s) in the formation of the precipitate, the p b2 and i − ions combine to form insoluble p bi 2 . the other ions, k and n o3− , do not participate in the formation of the precipitate. they remain in solution and are therefore called spectator ions. To determine the correct balanced net ionic equation for the given precipitation reaction, we need to first write the complete ionic equation and then. This is a double displacement reaction, so the products will be formed by exchanging the anions of the reactants: pb (no3)2 (aq) ki (aq) → pbi2 (s) kno3 (aq) now, we need to balance the equation. This method separates the reaction into two half reactions – one for oxidation and one for reduction. each half reaction is balanced separately and then combined.

Solved Example Pb No3 2 Aq 2 Kl Aq 2 Kno3 Aq Chegg This is a double displacement reaction, so the products will be formed by exchanging the anions of the reactants: pb (no3)2 (aq) ki (aq) → pbi2 (s) kno3 (aq) now, we need to balance the equation. This method separates the reaction into two half reactions – one for oxidation and one for reduction. each half reaction is balanced separately and then combined. Our expert help has broken down your problem into an easy to learn solution you can count on. there are 2 steps to solve this one. Find chemistry textbook solutions? still have questions?. Question 8 for the reaction: pb (no3)2 (aq) 2 kl (aq) > pbl2 (s) 2 kno3 (aq) you have 12 g of pb (no3)2 and 19 g of ki after the reaction is complete, you weigh your product and determine actual yield as 11 g. Here’s the best way to solve it. the balanced chemical reaction is as follows: pb (no3)2 (aq) 2ki (aq) pbi2 (s) 2kno3 (aq) [pb (no3)2] = 0.706 m the volume of pb (no3)2 = 50.0 ml x ( 1 l 1000 ml) = 0.05 l [ki] = 0.402 m volume of ki = 125.0 ml x ( 1 l 1000 ml) = 0.125 l determine t ….
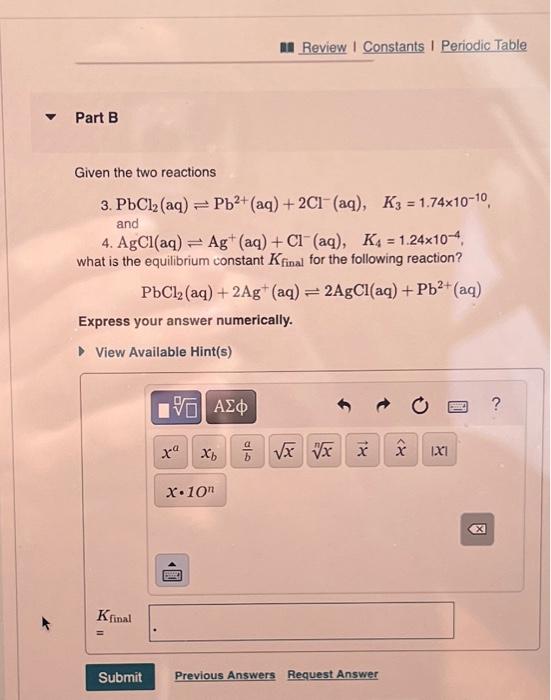
Solved Part B Given The Two Reactions 3 Pbcl2 Aq Pb Chegg Our expert help has broken down your problem into an easy to learn solution you can count on. there are 2 steps to solve this one. Find chemistry textbook solutions? still have questions?. Question 8 for the reaction: pb (no3)2 (aq) 2 kl (aq) > pbl2 (s) 2 kno3 (aq) you have 12 g of pb (no3)2 and 19 g of ki after the reaction is complete, you weigh your product and determine actual yield as 11 g. Here’s the best way to solve it. the balanced chemical reaction is as follows: pb (no3)2 (aq) 2ki (aq) pbi2 (s) 2kno3 (aq) [pb (no3)2] = 0.706 m the volume of pb (no3)2 = 50.0 ml x ( 1 l 1000 ml) = 0.05 l [ki] = 0.402 m volume of ki = 125.0 ml x ( 1 l 1000 ml) = 0.125 l determine t ….

Solved Part B Given The Two Reactions 3 Pbcl Aq Pb Chegg Question 8 for the reaction: pb (no3)2 (aq) 2 kl (aq) > pbl2 (s) 2 kno3 (aq) you have 12 g of pb (no3)2 and 19 g of ki after the reaction is complete, you weigh your product and determine actual yield as 11 g. Here’s the best way to solve it. the balanced chemical reaction is as follows: pb (no3)2 (aq) 2ki (aq) pbi2 (s) 2kno3 (aq) [pb (no3)2] = 0.706 m the volume of pb (no3)2 = 50.0 ml x ( 1 l 1000 ml) = 0.05 l [ki] = 0.402 m volume of ki = 125.0 ml x ( 1 l 1000 ml) = 0.125 l determine t ….
Comments are closed.