Solved Question 13 1 Pts Consider An Unbalanced Molecular Chegg
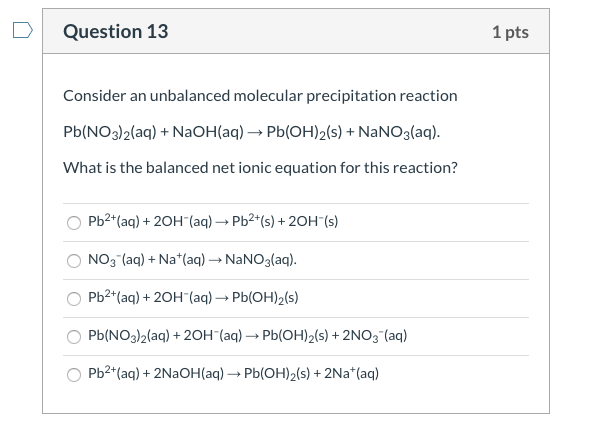
Solved Question 13 1 Pts Consider An Unbalanced Molecular Chegg Question: question 13 1 pts consider an unbalanced molecular precipitation reaction pb (no3)2 (aq) naoh (aq) → pb (oh)2 (s) nano3 (aq). what is the balanced net ionic equation for this reaction?. In a laboratory synthesis, a student begins with 3.00 ml of acetic anhydride (density = 1.08 g ml) and 1.25 g of salicylic acid. once the reaction is complete, the student collects 1.22 g of aspirin. determine the limiting reactant, theoretical yield of aspirin, and percent yield for the reaction.

Solved Consider The Following Unbalanced Molecular Equation Chegg Consider the unbalanced molecular equation for a redox reaction shown below. match each species in the reaction with the correct description of the role it plays. li(s) hno3(aq) ? li(no3)2(aq) h2(g) li(s) drop zone empty. li (aq) drop zone empty. no3 (aq) drop zone empty. h (aq) drop zone empty. h2(g) drop zone empty. oops!. Understand the molecular image of the given unbalanced chemical reaction and write the unbalanced chemical equation based on it. Consider the following unbalanced particulate representation of a chemical equation: h = light blue i = purple write a balanced chemical equation for this reaction, using the smallest integer coefficients possible. do not include states. Consider the unbalanced chemical reaction in the molecular image shown below. the creamy colored spheres represent hydrogen atoms and the blue ones represent oxygen atoms.
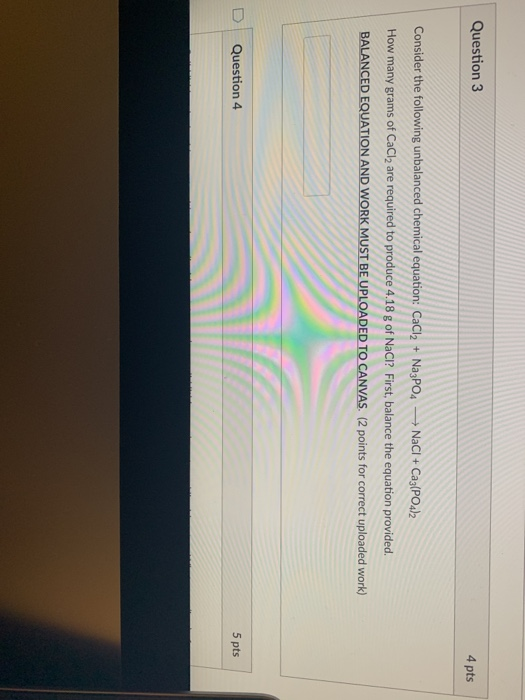
Solved Question 3 4 Pts Consider The Following Unbalanced Chegg Consider the following unbalanced particulate representation of a chemical equation: h = light blue i = purple write a balanced chemical equation for this reaction, using the smallest integer coefficients possible. do not include states. Consider the unbalanced chemical reaction in the molecular image shown below. the creamy colored spheres represent hydrogen atoms and the blue ones represent oxygen atoms. Consider the following unbalanced particulate representation of a chemical equation: h = light blue o o i = purple write a balanced chemical equation for this reaction, using the smallest integer coefficients possible. Question: question 11 12 pts consider the following unbalanced molecular equation: kaslag) pb (no3)2 (aq) pb (s) knoxlaq) after balancing this equation, write the lonic equation and the net ionic equation. To balance the chemical equation, place coefficients in front of the chemical formulas so that the number of atoms of each element is equal on both sides of the equation. it is clear that the given equation lacks balance regarding the number of fluorine atoms. Question: consider the unbalanced chemical reaction in the molecular amage shown below. the red spheres are for calcium ions, the blue spheres are for hydrogen atoms and the green spheres are for oxygen atoms.
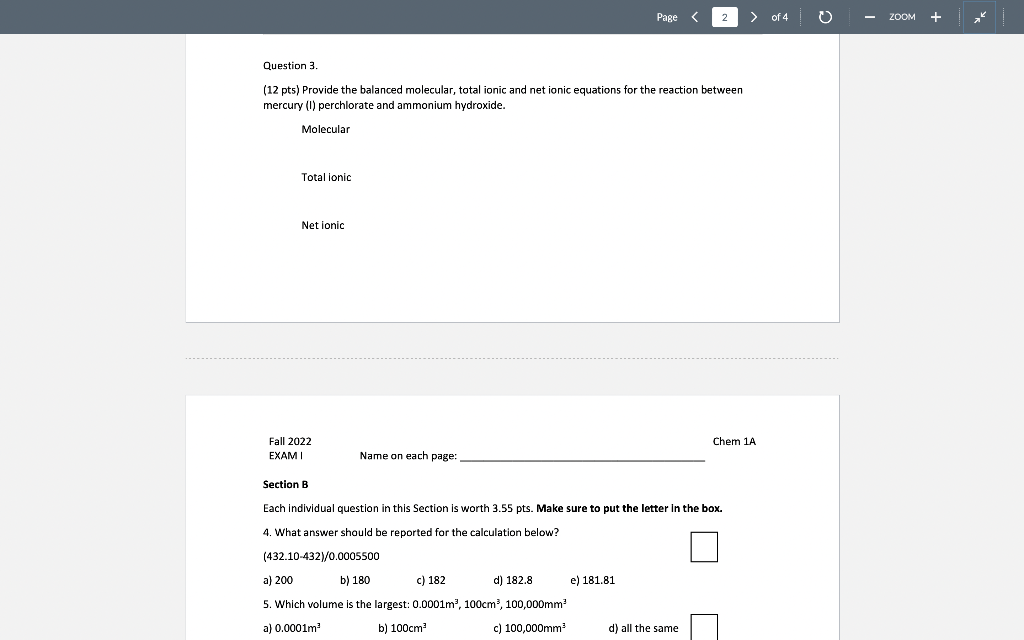
Solved Question 3 12 Pts Provide The Balanced Molecular Chegg Consider the following unbalanced particulate representation of a chemical equation: h = light blue o o i = purple write a balanced chemical equation for this reaction, using the smallest integer coefficients possible. Question: question 11 12 pts consider the following unbalanced molecular equation: kaslag) pb (no3)2 (aq) pb (s) knoxlaq) after balancing this equation, write the lonic equation and the net ionic equation. To balance the chemical equation, place coefficients in front of the chemical formulas so that the number of atoms of each element is equal on both sides of the equation. it is clear that the given equation lacks balance regarding the number of fluorine atoms. Question: consider the unbalanced chemical reaction in the molecular amage shown below. the red spheres are for calcium ions, the blue spheres are for hydrogen atoms and the green spheres are for oxygen atoms.
Comments are closed.