Solved Question 2 1pts Balance The Following Reaction And Chegg
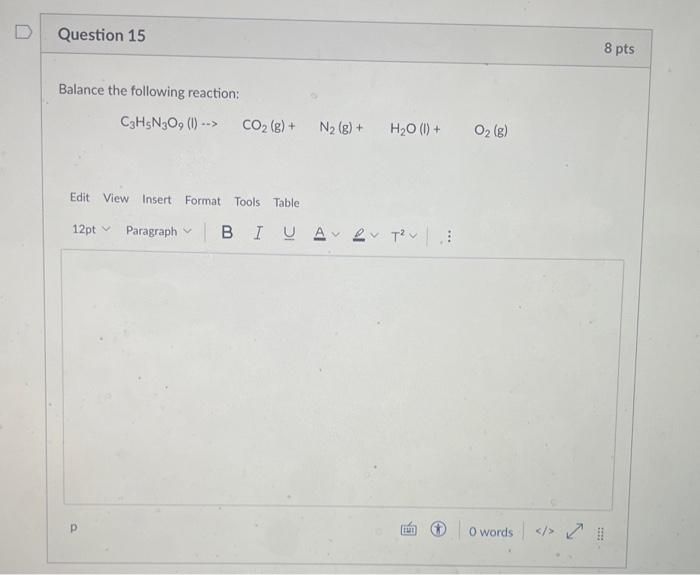
Solved Balance The Following Reaction Chegg Question: question 2 1pts balance the following reaction and calculate how many moles of no2 form when 1.2 moles of the reactant completely react. Study with quizlet and memorize flashcards containing terms like what numbers belong in the blank spaces to fully balance the following reaction?.

Solved Balance The Following Reaction From The Equation In Chegg Balancing redox reactions in basic solution involves several steps. here's how you can balance the given reaction: step 1: separate the reaction into half reactions the reaction. what is the equilibrium constant for the following reaction at 25 degrees celcius?. Any violation of the law of conservation of matter has been avoided. the chemical equation for this reaction above would be written as: 2 h 2 (g) o 2 (g) → 2 h 2 o (g) this form of the chemical equation is called a balanced chemical equation. a balanced chemical equation is one that conforms to the law of conservation of matter. Balance the following redox reaction using the steps: 1) split into half reactions, 2) balance all except o and h, 3) balance o using h20, 4) balance h using h', 5) balance charges using e , 6) balance e flow between half reactions, 7) sum the half reactions then cancel like terms. Balance any equation or reaction using this chemical equation balancer! find out what type of reaction occured.
Solved Balance The Following Reaction From The Equation In Chegg Balance the following redox reaction using the steps: 1) split into half reactions, 2) balance all except o and h, 3) balance o using h20, 4) balance h using h', 5) balance charges using e , 6) balance e flow between half reactions, 7) sum the half reactions then cancel like terms. Balance any equation or reaction using this chemical equation balancer! find out what type of reaction occured. Example #11: balance the equation for the reaction of stannous ion with pertechnetate in acidic solution. products are stannic ion, sn 4 and technetium (iv), tc 4 ions. For each problem, list the necessary coefficients in order to balance the equations. learn with flashcards, games, and more — for free. Balance each redox reactions occurring in basic aqueous solution. express your answer as a chemical equation. identify all of the phases in your answer. balancing redox reactions in basic solution involves several steps. here's how you can balance the given reaction: the given reaction is: i2(g) → i−(aq) io3−(aq). Redox reactions in basic aqueous solution (two parts) balance the following equations, using either the half reaction method or the oxidation number method, and enter the coefficients in the boxes.
Comments are closed.