Solved Show All Calculations 1 Determine The Ksp Chegg

Solved Show All Calculations 1 Determine The Ksp Chegg Example #1: a solid sample of ca (oh) 2 is shaken with 0.0100 m cacl 2. once equilibrated, some solid ca (oh) 2 remains undissolved. the solution is filtered and a 25.00 ml sample requires 22.50 ml of 0.0250 m hcl to neutralize it. calculate the value for k sp of ca (oh) 2 from this data. Determine the concentrations of all ions in solution when the solutions are mixed and use them to calculate the ion product (q). compare the values of q and ksp to decide whether a precipitate will form.
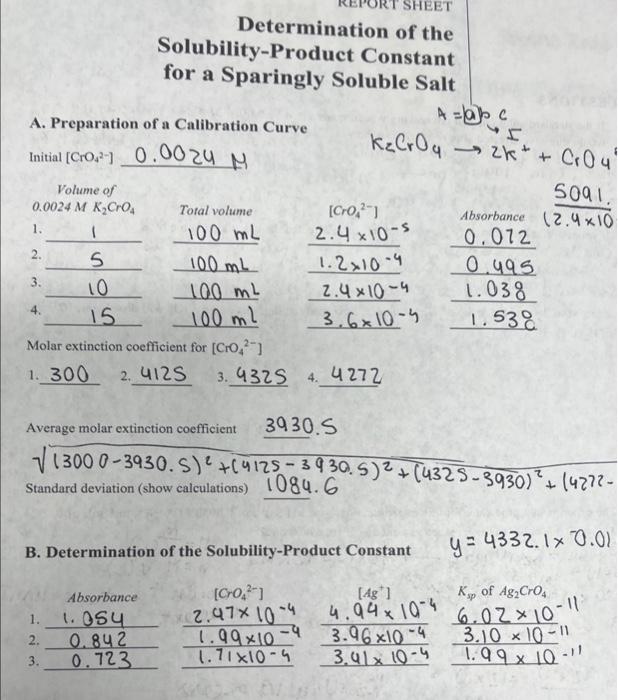
Solved Give Me The Average Ksp Show Calculations And The Chegg Find the accepted value of the ksp for calcium hydroxide and compare it with your value. discuss the discrepancy and suggest possible sources of experimental error. What is the minimum ph required for precipitation? the reaction quotient: will a precipitate form?. Learn to calculate ksp, solubility, and compare ksp values. chemistry worksheet for high school early college students. In order to find the ksp value, the hydroxide concentration will have to be determined in the slightly soluble salt concentration set up during the experiment. the saturated solution consists of the maximum amount of solute that should dissolve in the solution.

Solved Calculations 1 Show Complete Calculations To Chegg Learn to calculate ksp, solubility, and compare ksp values. chemistry worksheet for high school early college students. In order to find the ksp value, the hydroxide concentration will have to be determined in the slightly soluble salt concentration set up during the experiment. the saturated solution consists of the maximum amount of solute that should dissolve in the solution. Calculate the molar solubility of ca(oh)2 at 50 ̊c using your experimental values of Δh ̊ and Δs ̊. (hint: first find Δg ̊ at the new temperature, then determine ksp and finally solubility.).
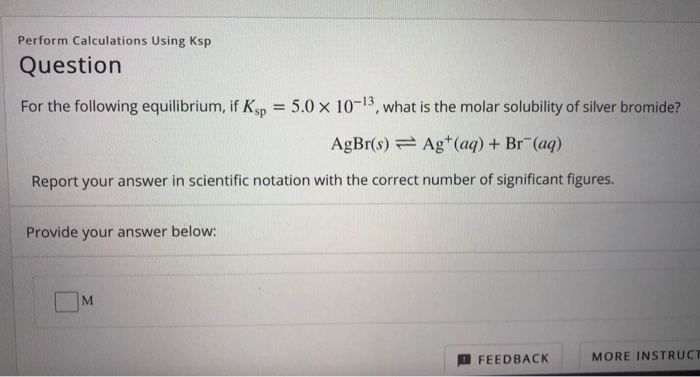
Solved Perform Calculations Using Ksp Question For The Chegg Calculate the molar solubility of ca(oh)2 at 50 ̊c using your experimental values of Δh ̊ and Δs ̊. (hint: first find Δg ̊ at the new temperature, then determine ksp and finally solubility.).
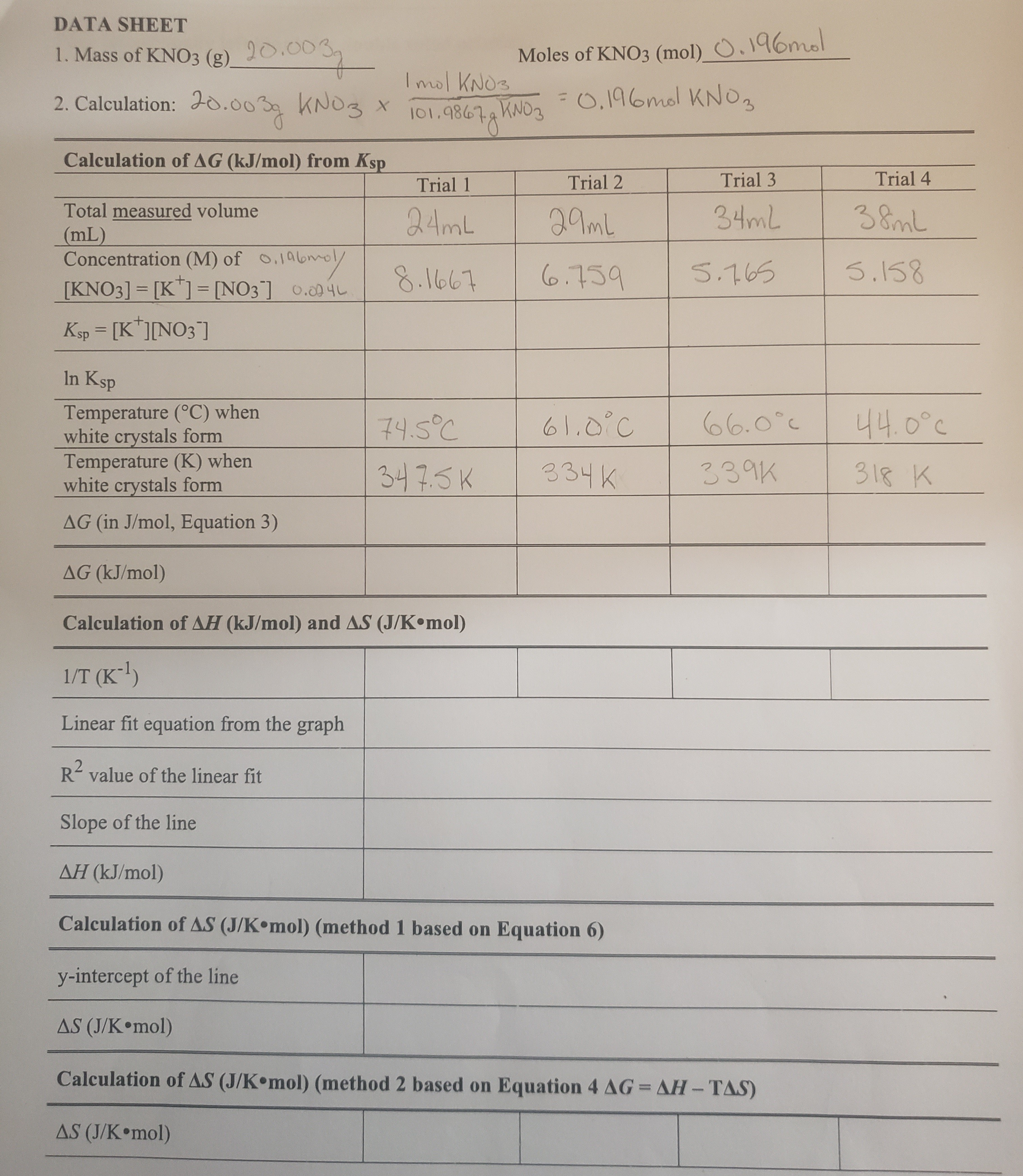
Solved How To Calculate Ksp Based On My Calculations Are Chegg
Comments are closed.