Solved The Equation Below Shows The Decomposition Of Lead Nitrate How
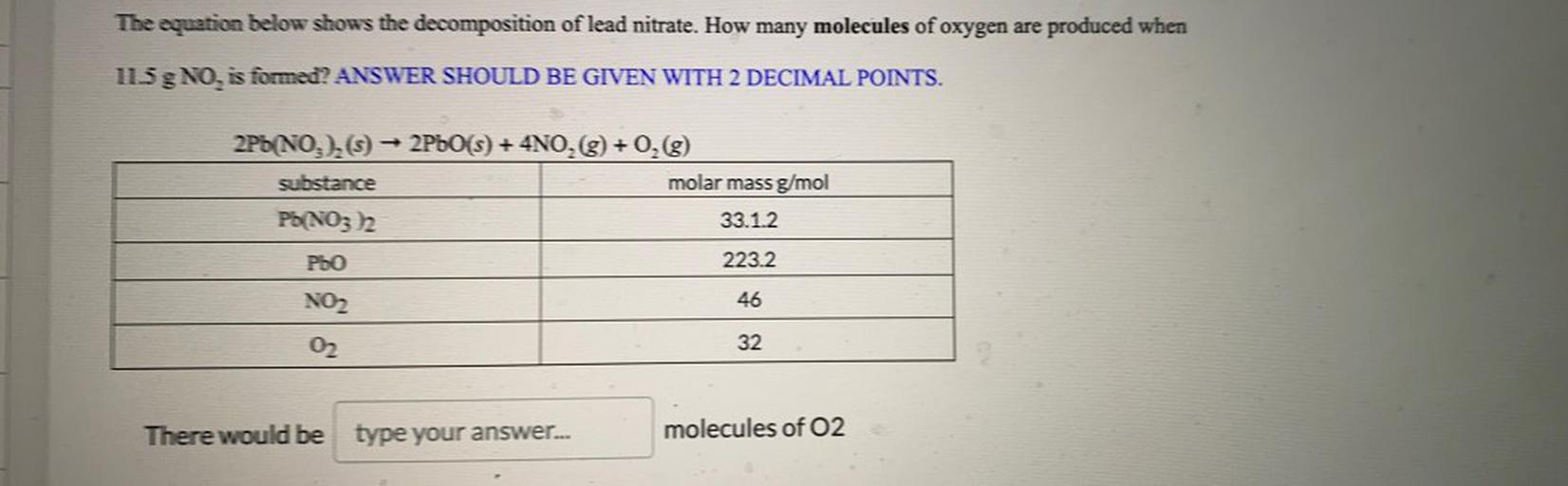
The Equation Below Shows The Decompositio Physical Chemistry This calculation utilizes established principles of stoichiometry in chemistry, including the concept that balanced chemical equations provide mole ratios that can be used to convert between different substances. The equation below shows the decomposition of lead nitrate. how many grams of oxygen are produced when 11.5g no2 is formed? 2pb (no3)2 > 2pbo 4no2 o2.
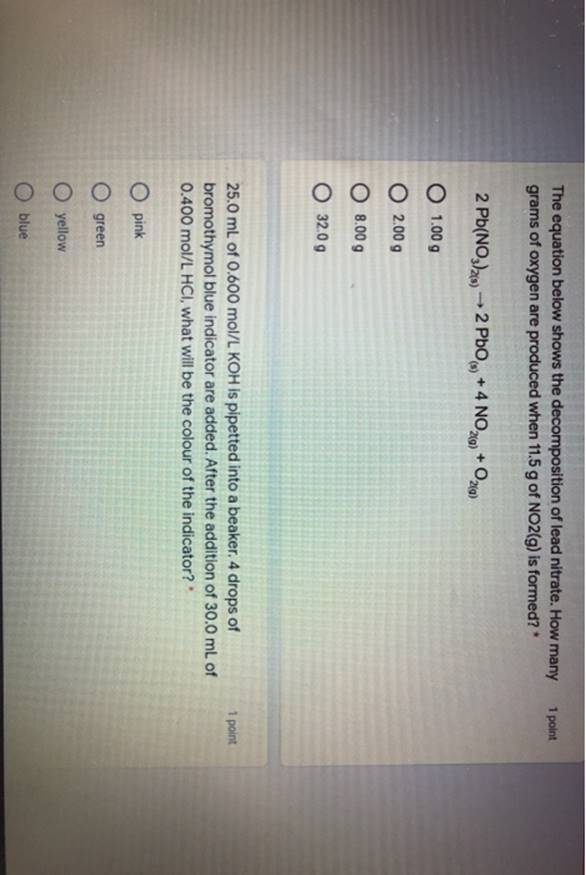
Solved The Equation Below Shows The Decomposition Of Lead Nitrate Answer the balanced chemical equation for the decomposition of lead nitrate is: 2 pb(no3)2 > 2 pbo 4 no2 o2 from this equation, we can see that 1 mole of o2 is produced for every 4 moles of no2. first, we need to convert the mass of oxygen to moles. the molar mass of o2 is approximately 32.0 g mol. To determine the number of grams of oxygen produced when 11.5 g of no2 is formed in the decomposition of lead nitrate, we need to use the stoichiometry of the reaction. There are 2 steps to solve this one. stoichiometry in chemistry deals with the quantitative relationships in chemical reactions. it invol the equation below shows the decomposition of lead nitrate. Write down the balanced chemical equation for the decomposition of lead nitrate. the equation is already given as: \ [2pb (no 3) 2 (s) \rightarrow 2pbo (s) 4no 2 (g) o 2 (g)\].
Solved The Equation Below Shows The Decomposition Of Lead Nitrate How There are 2 steps to solve this one. stoichiometry in chemistry deals with the quantitative relationships in chemical reactions. it invol the equation below shows the decomposition of lead nitrate. Write down the balanced chemical equation for the decomposition of lead nitrate. the equation is already given as: \ [2pb (no 3) 2 (s) \rightarrow 2pbo (s) 4no 2 (g) o 2 (g)\]. To solve this problem, we first need to understand the decomposition reaction of lead nitrate (pb (no3)2). the balanced equation for the decomposition is: 2 pb (no3)2 → 2 pbo 4 no2 o2. from the balanced equation, we can see the following molar ratios: 4 moles of no2 are produced for every 1 mole of o2 produced. Use the stoichiometry of the reaction to find the number of moles of $$o {2}$$o2 produced.from the balanced equation, the mole ratio between $$no {2}$$no2 and $$o {2}$$o2 is 4:1.number of moles of $$o {2}$$o2 = 0.2500 mol × (1 mol $$o {2}$$o2 4 mol $$no {2}$$no2 ) = 0.0625 mol. Question: the equation below shows the decomposition of lead nitrate. how many grams of pbo are produced when 8.4 g 0, is formed? 2pb (no), 2pbo 4no, o, your answer. Answer to solve this problem, we need to use the concept of stoichiometry, which is the calculation of relative quantities of reactants and products in chemical reactions.
Comments are closed.