Solved The Following Equation Is Not Balanced Pb No3 2 Chegg

Solved Consider The Following Balanced Chemical Equation Chegg Question: the following equation is not balanced pb (no3)2 nacl pbch nano part a notice that no," appears in two different places in this chemical equation. no, is a polyatomic ion called nitrate. Video answer: this is sort of a balanced equation where aluminum nitrates reacts with a sulfide. this is going to make a matrix of….
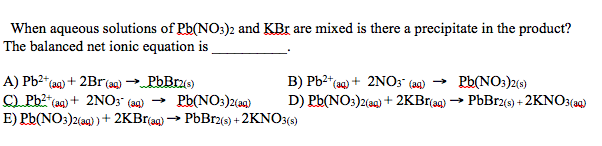
Solved When Aqueous Solutions Of Pb No3 2 And Kbr Are Mixed Chegg What number should be placed in front of nano3 to give the same total number of nitrate ions on each side of the equation? (express answer numerically as an integer). The elements that are not balanced in the equation are nitrogen (n), potassium (k), and oxygen (o). to balance the equation, modify coefficients, such as putting a 2 in front of kno₃. For each element that is not equal, try to balance it by adding more of it to the side with less. sometimes there may be multiple compounds with that element on one side, so you'll need to use your best judgement and be prepared to go back and try the other options. Predict the products of the following reactions and then balance the equation. make sure to put in the phases of the products. pb (no3)2 (aq) na3po4 (aq) → a. for the above equation, write the complete ionic and net ionic equations. b. if you have a 2.5 m solution of lead (ii) nitrate, and a 3.5 m solution of sodium phosphate, how much of.

Solved The Following Equation Is Not Balanced Pb No3 2 Chegg For each element that is not equal, try to balance it by adding more of it to the side with less. sometimes there may be multiple compounds with that element on one side, so you'll need to use your best judgement and be prepared to go back and try the other options. Predict the products of the following reactions and then balance the equation. make sure to put in the phases of the products. pb (no3)2 (aq) na3po4 (aq) → a. for the above equation, write the complete ionic and net ionic equations. b. if you have a 2.5 m solution of lead (ii) nitrate, and a 3.5 m solution of sodium phosphate, how much of. Give a chemical test to distinguish between the following gases. write your observation and a balanced equation in the case of the following substances being heated. copper carbonate. explain the term ‘chemical reaction’ with special reference to – ‘reactants’ and ‘products’. Science chemistry chemistry questions and answers balance the following oxidation reduction reaction in basic solution no3 pb — > no pb 2. A more complex problem: the common ion effect calculating the solubility of pb (io 3) 2 in deionized water is a straightforward problem because the solid’s dissolution is the only source of pb 2 and \ (\text {io} 3^ \). but what if we add pb (io 3) 2 to a solution of 0.10 m pb (no 3) 2? before we set‐up and solve this problem algebraically, think about the system’s chemistry and decide. The student proposed the balanced chemical equation for the reaction between lead (ii) nitrate and sodium iodide as pb (no3)2 2nai → pbi2 2nano3. to determine if this is a correctly balanced equation, we can count the atoms of each element on both sides of the equation.

Solved 3 For The Balanced Reaction Pb No3 2 2nacl Chegg Give a chemical test to distinguish between the following gases. write your observation and a balanced equation in the case of the following substances being heated. copper carbonate. explain the term ‘chemical reaction’ with special reference to – ‘reactants’ and ‘products’. Science chemistry chemistry questions and answers balance the following oxidation reduction reaction in basic solution no3 pb — > no pb 2. A more complex problem: the common ion effect calculating the solubility of pb (io 3) 2 in deionized water is a straightforward problem because the solid’s dissolution is the only source of pb 2 and \ (\text {io} 3^ \). but what if we add pb (io 3) 2 to a solution of 0.10 m pb (no 3) 2? before we set‐up and solve this problem algebraically, think about the system’s chemistry and decide. The student proposed the balanced chemical equation for the reaction between lead (ii) nitrate and sodium iodide as pb (no3)2 2nai → pbi2 2nano3. to determine if this is a correctly balanced equation, we can count the atoms of each element on both sides of the equation.
Comments are closed.