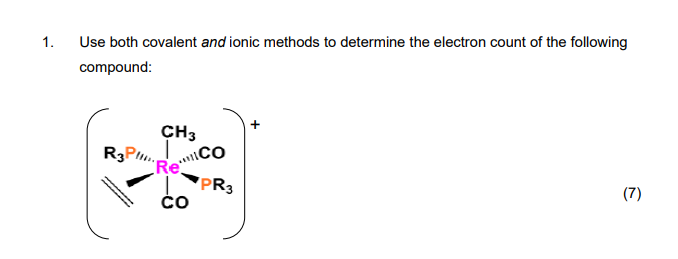
Solved Use Both Covalent And Ionic Methods To Determine The Chegg Our expert help has broken down your problem into an easy to learn solution you can count on. there are 2 steps to solve this one. if there are two co ligands (carbonyl groups) in the [re (co) (co) (ch 3) (pr 3) (pr 3) (ch 2 ch 2)] complex, l not the question you’re looking for? post any question and get expert help quickly. Question: use both the covalent and ionic method to determine the electron count of each of the following complexes:1.2.3.4.5. [au(oh2)2cl4]2 6. ,[(η3 cp)co(oh2)4(me)].

Solved Ionic Or Covalent Determine If The Compounds Are Chegg To find the number of electrons in ferrocene using the covalent method, add the number of valence electrons of the iron (fe) atom to the total number of electrons contributed by the two ligands. view the full answer. There are two widely used methods for electron counting of complexes covalent method and ionic ligand method. both of the two methods are applicable to all organometallic complexes, and should give the same electron count. in this method, all metal ligand bonds are considered covalent. Determine whether each of the following compounds would be best represented by an ionic or a covalent lewis structure. use lewis theory to determine the formula for the compound that forms from mg and br. Using the periodic table, predict whether the following chlorides are ionic or covalent: sicl 4, pcl 3, cacl 2, cscl, cucl 2, and crcl 3. for each of the following compounds, state whether it is ionic or covalent. if it is ionic, write the symbols for the ions involved:.

Lab 2 Ionic And Covalent Pdf Solubility Chemical Compounds Determine whether each of the following compounds would be best represented by an ionic or a covalent lewis structure. use lewis theory to determine the formula for the compound that forms from mg and br. Using the periodic table, predict whether the following chlorides are ionic or covalent: sicl 4, pcl 3, cacl 2, cscl, cucl 2, and crcl 3. for each of the following compounds, state whether it is ionic or covalent. if it is ionic, write the symbols for the ions involved:. Study with quizlet and memorize flashcards containing terms like bonding, ionic bonds, covalent bonds and more. Use the data to determine whether the substances likely have ionic or covalent bonds. record your conclusions in the last row of the data table. (you may be asked later to justify your conclusions.). Our expert help has broken down your problem into an easy to learn solution you can count on. question: use both the ionic and covalent counting systems to give electron counts for the metal and each type of ligand. use these to determine the total electron count for these organometallic compounds (watch for oxidation state of cationic complexes!. Study with quizlet and memorize flashcards containing terms like classify each solid as a covalent, ionic, metallic, or molecular solid., classify each of the following materials as molecular, metallic, ionic, or covalent network., which type (or types) of crystalline solid is characterized by each of the following. and more.

Lab 4 Investigating Ionic And Covalent Compounds Pdf Chemical Study with quizlet and memorize flashcards containing terms like bonding, ionic bonds, covalent bonds and more. Use the data to determine whether the substances likely have ionic or covalent bonds. record your conclusions in the last row of the data table. (you may be asked later to justify your conclusions.). Our expert help has broken down your problem into an easy to learn solution you can count on. question: use both the ionic and covalent counting systems to give electron counts for the metal and each type of ligand. use these to determine the total electron count for these organometallic compounds (watch for oxidation state of cationic complexes!. Study with quizlet and memorize flashcards containing terms like classify each solid as a covalent, ionic, metallic, or molecular solid., classify each of the following materials as molecular, metallic, ionic, or covalent network., which type (or types) of crystalline solid is characterized by each of the following. and more.
