Solved What Is The Precipitate In The Following Reaction Bacl2 Aq
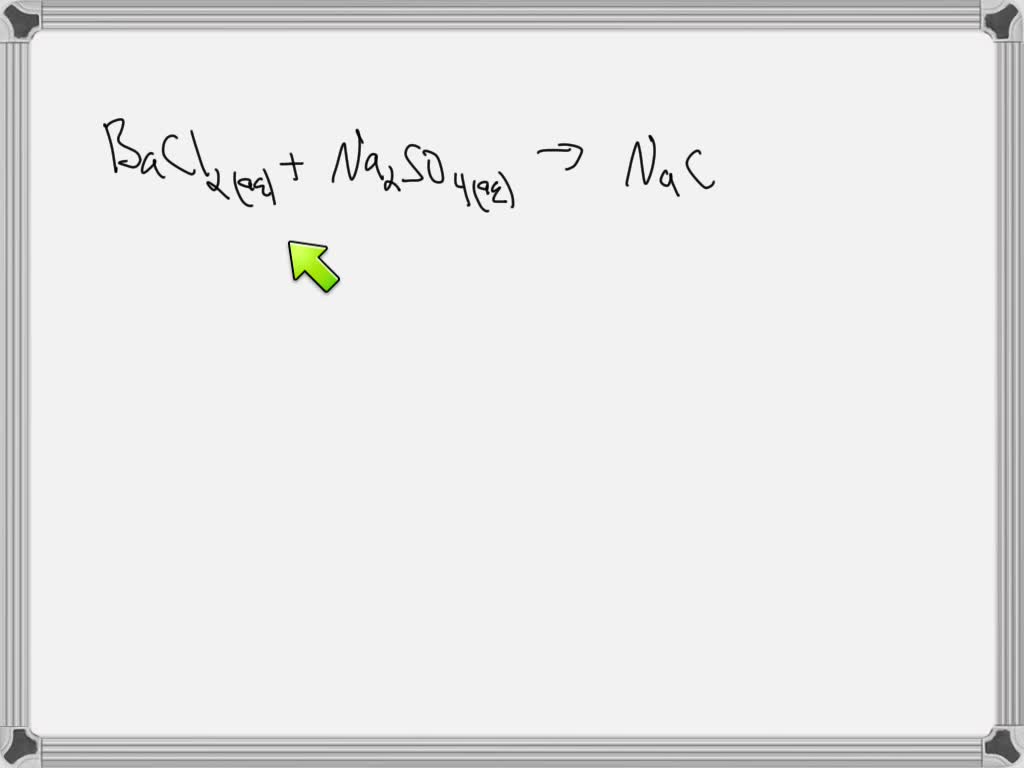
Solved What Is The Precipitate In The Following Reaction Bacl2 Aq The precipitate formed in the reaction between bacl₂ and na₂so₄ is baso₄, which is insoluble in water. therefore, the correct answer is b. baso₄. understanding solubility rules helps to predict the formation of precipitates in such reactions. Upon mixing a clear colorless bacl2 solution with a clear, colorless na2so4 solution according to the reaction below, a student observes a white cloudiness form. what is responsible for the cloudiness that is observed?bacl2 (aq) na2so4 (aq) → baso4 (s) 2nacl (aq).
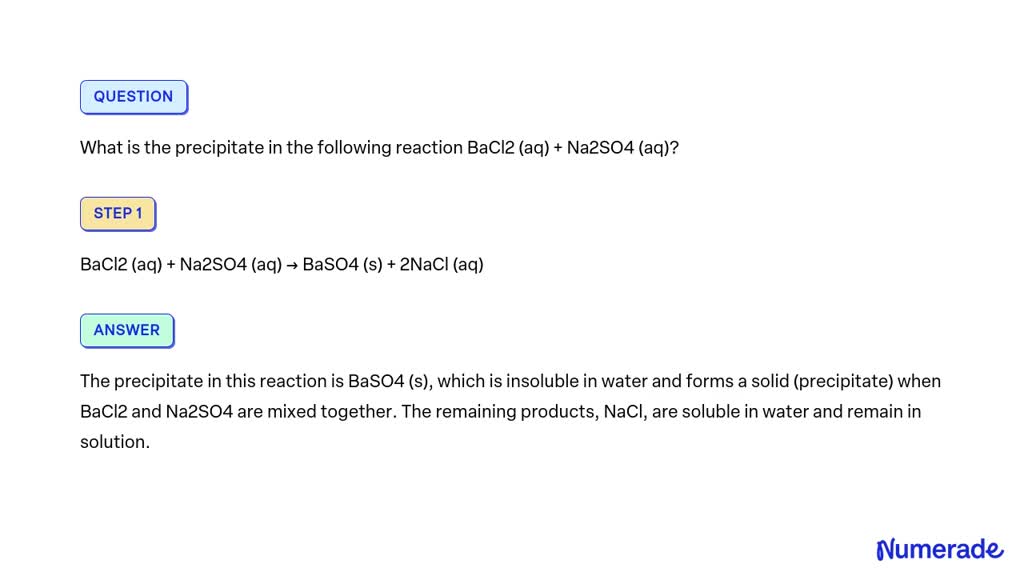
Solved What Is The Precipitate In The Following Reaction Bacl2 Aq A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. thus precipitation reactions are a subclass of exchange reactions that …. Question: refer to the precipitation reaction below. bacl2 (aq) h2so4 (aq) → baso4 (s) 2hcl (aq) how much 0.22 m h2so4 solution in liters will completely precipitate the ba2 ion in 1.2 l of 0.19 m bacl2 solution?. In order to identify the precipitate in the following reaction, we're going to have to reference the solubility rules. according to the solubility rules, barium chloride is soluble, and sodium sulfate is also soluble, so we write both of these as aqueous. Bacl₂ (aq) h₂so₄ (aq) → baso₄ (s) 2hcl (aq) this shows a 1:1 molar ratio between bacl₂ and h₂so₄, meaning one mole of sulfuric acid reacts with one mole of barium chloride to form one mole of barium sulfate precipitate.
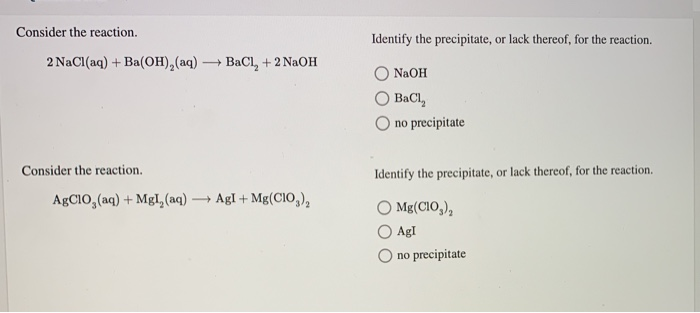
Solved Consider The Reaction Identify The Precipitate Or Chegg In order to identify the precipitate in the following reaction, we're going to have to reference the solubility rules. according to the solubility rules, barium chloride is soluble, and sodium sulfate is also soluble, so we write both of these as aqueous. Bacl₂ (aq) h₂so₄ (aq) → baso₄ (s) 2hcl (aq) this shows a 1:1 molar ratio between bacl₂ and h₂so₄, meaning one mole of sulfuric acid reacts with one mole of barium chloride to form one mole of barium sulfate precipitate. In this reaction, barium chloride and silver nitrate are mixed in aqueous solution. the barium ion (ba²⁺) and the silver ions (ag⁺) exchange their anions, resulting in the formation of silver chloride (agcl), which is a solid precipitate that forms and settles out of the solution. Yes, a precipitate will form when sodium hydroxide (naoh) is added to barium chloride (bacl2). the reaction between these two compounds results in the formation of barium hydroxide and sodium chloride. however, barium hydroxide is insoluble in water and will therefore form a white precipitate. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. the complete chemical equation can be written to describe what happens, and such an equation is useful in making chemical calculations. The precipitate formed when bacl2 and k2so4 are mixed is baso4 (barium sulfate), according to the balanced chemical reaction: bacl2 (aq) k2so4 (aq) → baso4 (s) 2 kcl (aq).
Comments are closed.