
Soraya Data Shed Light On Responses To Mirvetuximab Soravtansine Based Clinicians now have more nuanced information on how the antibody drug conjugate mirvetuximab soravtansine (elahere, immunogen) works in patients with folate receptor alpha high (frα high). Treatment with the folate receptor alpha (frα) targeted antibody drug conjugate mirvetuximab soravtansine gynx benefited patients with frα high, platinum resistant ovarian cancer, even after multiple lines of prior therapy, according to an updated analysis of the soraya trial presented at the society of gynecologic oncology (sgo) 2023 annual.

On Immunogen And Mirvetuximab Soravtansine Imgn 853 A Subjective Mirvetuximab soravtansine (mirv) is an antibody drug conjugate targeting folate receptor α (frα). soraya is a single arm, phase ii study evaluating efficacy and safety of mirv in patients with proc. methods: soraya enrolled frα high patients with proc who had received one to three prior therapies, including required bevacizumab. the primary. Treatment with the folate receptor alpha (frα) targeted antibody drug conjugate mirvetuximab soravtansine (mirv) benefited patients with frα high platinum resistant ovarian cancer (proc) regardless of the number of prior lines of therapy, according to new data from the soraya study. however, the drug yielded higher response rates as a first. Background: soraya is a global single arm phase 3 study evaluating mirv in patients (pts) with frα high platinum resistant ovarian cancer (proc). mirv is an antibody drug conjugate comprising a frα binding antibody, cleavable linker, and maytansinoid dm4, a potent tubulin targeting agent. Mirvetuximab soravtansine (mirv) is a biomarker driven antibody drug conjugate targeting folate receptor alpha (frα). in platinum resistant ovarian cancer (proc), objective response rate was 44% (n = 94; 5 complete and 36 partial responses). treatment was efficacious across all frα expression levels, but further improved with higher frα expression.

Mirvetuximab Soravtansine Gynx Elahere邃 Immunogen Background: soraya is a global single arm phase 3 study evaluating mirv in patients (pts) with frα high platinum resistant ovarian cancer (proc). mirv is an antibody drug conjugate comprising a frα binding antibody, cleavable linker, and maytansinoid dm4, a potent tubulin targeting agent. Mirvetuximab soravtansine (mirv) is a biomarker driven antibody drug conjugate targeting folate receptor alpha (frα). in platinum resistant ovarian cancer (proc), objective response rate was 44% (n = 94; 5 complete and 36 partial responses). treatment was efficacious across all frα expression levels, but further improved with higher frα expression. Objective: the single arm, phase ii soraya trial (nct04296890) of mirvetuximab soravtansine gynx in folate receptor alpha (frα) high platinum resistant ovarian cancer (n=105 (efficacy evaluable)) met its primary endpoint with an objective response rate of 32.4% (95% ci, 23.6 to 42.2). here we report final soraya trial results for overall. Clinically meaningful responses were demonstrated in subgroup analyses evaluating the sequencing of mirvetuximab soravtansine gynx (elahere) in patients with folate receptor alpha–high. Mirvetuximab soravtansine (mirv) is a first in class adc comprising a folate receptor α (frα) binding antibody, cleavable linker, and maytansinoid dm4 payload. here, we present updated data from the single arm study soraya on the clinical benefit and safety of mirv in frα high platinum resistant ovarian cancer (proc). Positive top line data were seen from the phase 3 soraya trial (nct04296890), investigating both the safety and efficacy of mirvetuximab soravtansine (imgn853) monotherapy in patients with folate receptor α (frα)–high platinum resistant ovarian cancer who previously received bevacizumab (avastin), according to a press release from immunogen.
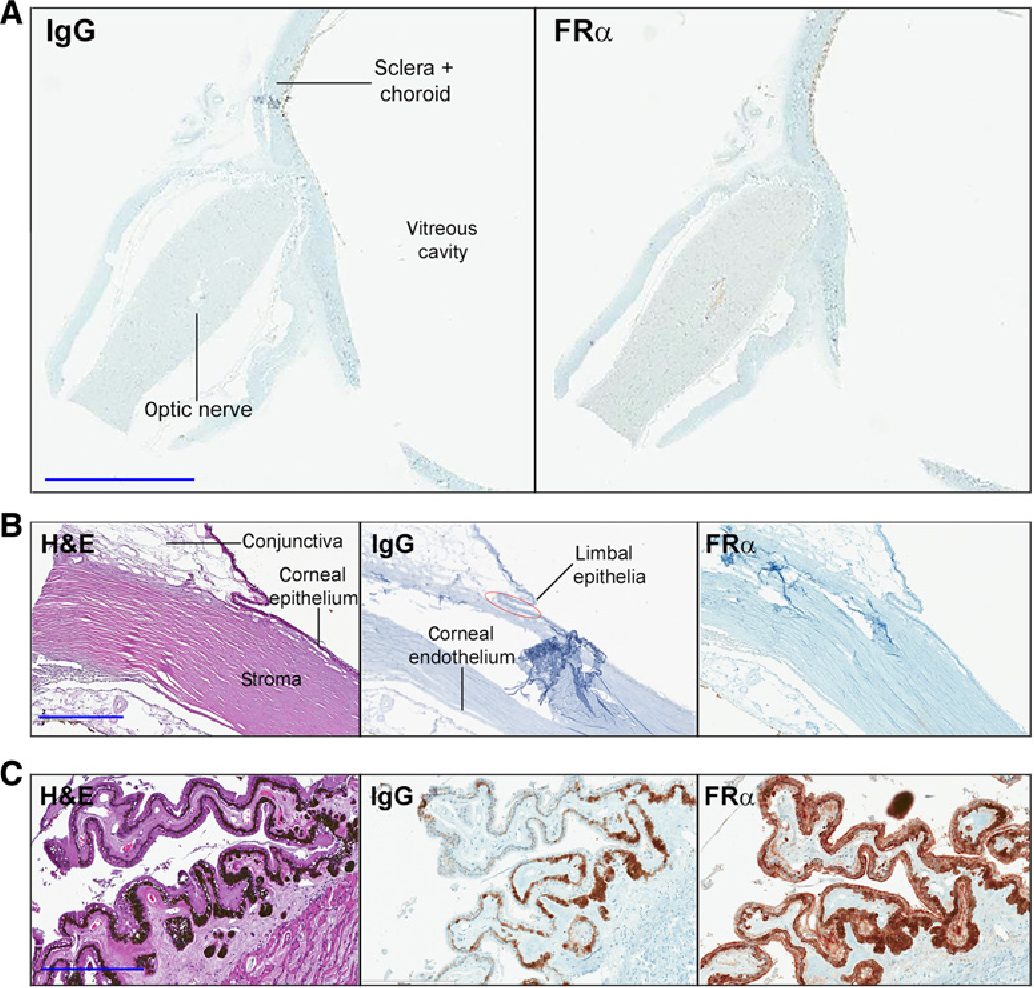
Mirvetuximab Soravtansine Semantic Scholar Objective: the single arm, phase ii soraya trial (nct04296890) of mirvetuximab soravtansine gynx in folate receptor alpha (frα) high platinum resistant ovarian cancer (n=105 (efficacy evaluable)) met its primary endpoint with an objective response rate of 32.4% (95% ci, 23.6 to 42.2). here we report final soraya trial results for overall. Clinically meaningful responses were demonstrated in subgroup analyses evaluating the sequencing of mirvetuximab soravtansine gynx (elahere) in patients with folate receptor alpha–high. Mirvetuximab soravtansine (mirv) is a first in class adc comprising a folate receptor α (frα) binding antibody, cleavable linker, and maytansinoid dm4 payload. here, we present updated data from the single arm study soraya on the clinical benefit and safety of mirv in frα high platinum resistant ovarian cancer (proc). Positive top line data were seen from the phase 3 soraya trial (nct04296890), investigating both the safety and efficacy of mirvetuximab soravtansine (imgn853) monotherapy in patients with folate receptor α (frα)–high platinum resistant ovarian cancer who previously received bevacizumab (avastin), according to a press release from immunogen.
