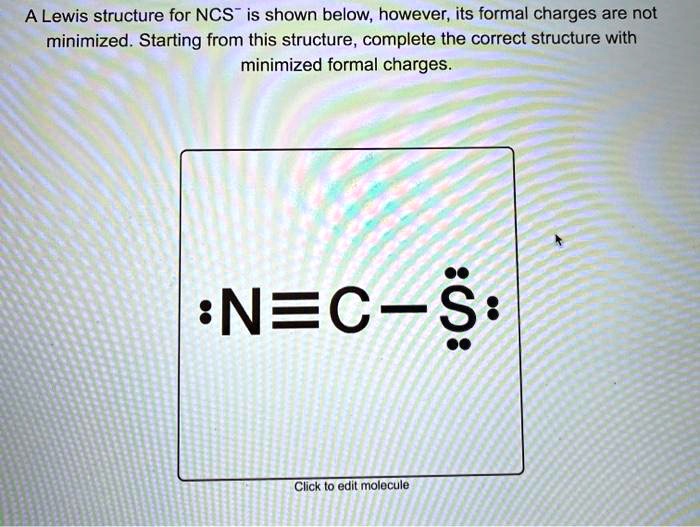
Get Answer A Lewis Structure For Ncs Is Shown Below However Its A lewis structure for ncs − is shown below, however, its formal charges are not minimized. starting from this structure, complete the minimized formal charges. : n ≡ c − ∘ s:. Cns is a chemical formula for thiocyanate ion. and to help you understand the lewis structure of this molecule, we are going to share our step by step meth.
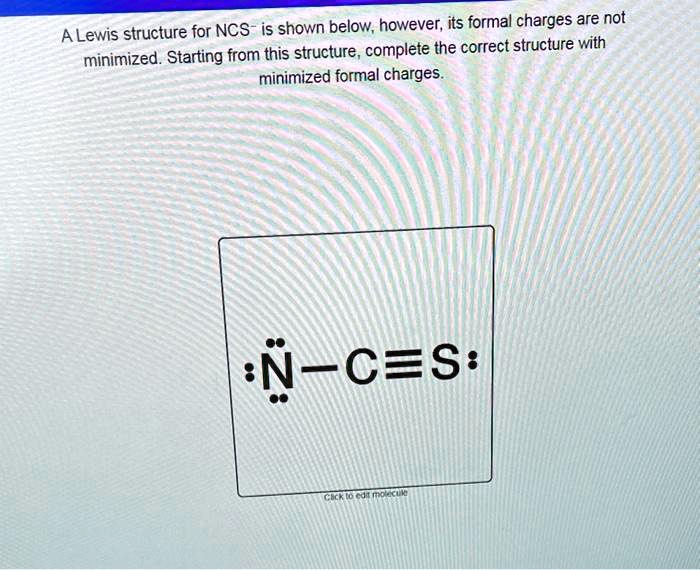
Structure For Ncs Is Shown Below However Its Formal Charges Are Not A The formal charges present in each of these molecular structures can help us pick the most likely arrangement of atoms. possible lewis structures and the formal charges for each of the three possible structures for the thiocyanate ion are shown here: note that the sum of the formal charges in each case is equal to the charge of the ion (–1). A lewis structure for ncs$^ $ is shown below, however, its formal charges are not minimized. starting from this structure, complete the correct structure with minimized formal charges.\ $:n \equiv c \ddot{s}:$. Two possible lewis structures for the cyanate ion are shown below, with formal charges (fc) calculated for each atom. one structure has carbon as the central atom and the other has a nitrogen as a central atom. by considering formal charges, which arrangement of atoms is the best, and therefore most likely structure for cyanate?. Video answer: in this problem, you are given this loose structure. and here i ask to find the minimized charge loose structure. so if we look at the formal charges on this molecule, if we look at nitrogen, it has five valence electrons.
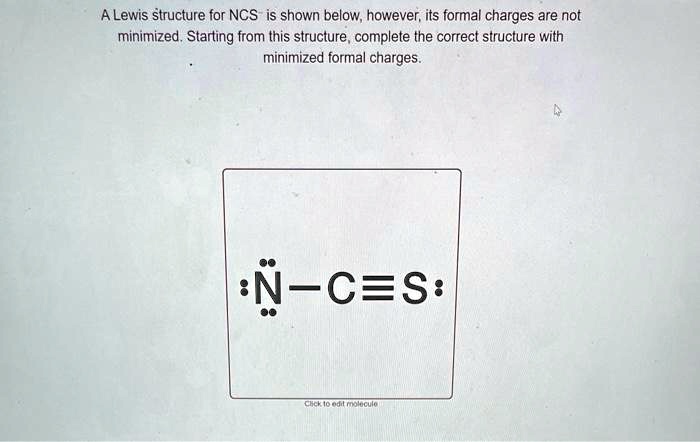
A Lewis Structure For Ncs Is Shown Below However Its Formal Charges Are Two possible lewis structures for the cyanate ion are shown below, with formal charges (fc) calculated for each atom. one structure has carbon as the central atom and the other has a nitrogen as a central atom. by considering formal charges, which arrangement of atoms is the best, and therefore most likely structure for cyanate?. Video answer: in this problem, you are given this loose structure. and here i ask to find the minimized charge loose structure. so if we look at the formal charges on this molecule, if we look at nitrogen, it has five valence electrons. In this final structure, the formal charges are minimized as follows: carbon (c): 0 formal charge; first oxygen (o): 1 formal charge; second oxygen (o): 1 formal charge; hydrogen (h): 0 formal charge; this arrangement of electrons and formal charges ensures the stability of the h₂co₃ molecule. Question: a lewis structure for ncs is shown below, however, its formal charges are not minimized. starting from this structure, complete the correct structure with minimized formal charges. ³n c=s: click to edit molecule. **task:** complete the correct lewis structure for ncs⁻ with minimized formal charges based on the structure provided. use the principles of formal charge calculation to adjust the electron distribution accordingly. Using formal charge to predict molecular structure. the arrangement of atoms in a molecule or ion is called its molecular structure.in many cases, following the steps for writing lewis structures may lead to more than one possible molecular structure—different multiple bond and lone pair electron placements or different arrangements of atoms, for instance.
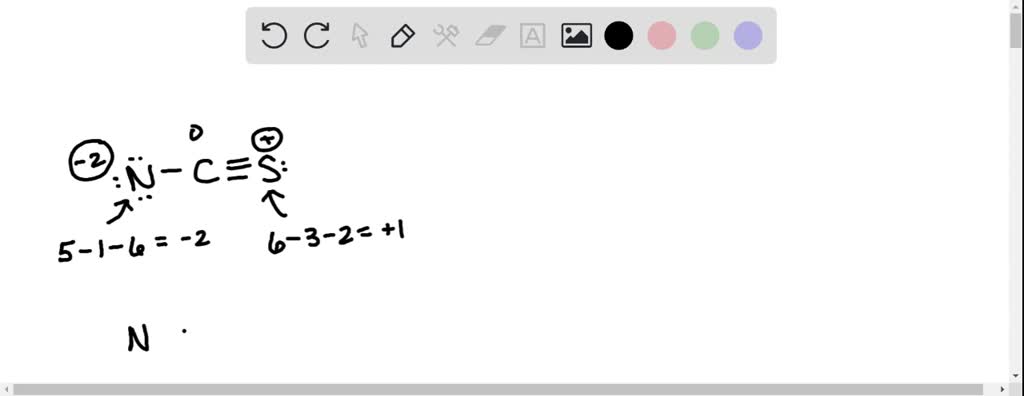
Structure For Ncs Is Shown Below However Its Formal Charges Are Not In this final structure, the formal charges are minimized as follows: carbon (c): 0 formal charge; first oxygen (o): 1 formal charge; second oxygen (o): 1 formal charge; hydrogen (h): 0 formal charge; this arrangement of electrons and formal charges ensures the stability of the h₂co₃ molecule. Question: a lewis structure for ncs is shown below, however, its formal charges are not minimized. starting from this structure, complete the correct structure with minimized formal charges. ³n c=s: click to edit molecule. **task:** complete the correct lewis structure for ncs⁻ with minimized formal charges based on the structure provided. use the principles of formal charge calculation to adjust the electron distribution accordingly. Using formal charge to predict molecular structure. the arrangement of atoms in a molecule or ion is called its molecular structure.in many cases, following the steps for writing lewis structures may lead to more than one possible molecular structure—different multiple bond and lone pair electron placements or different arrangements of atoms, for instance.
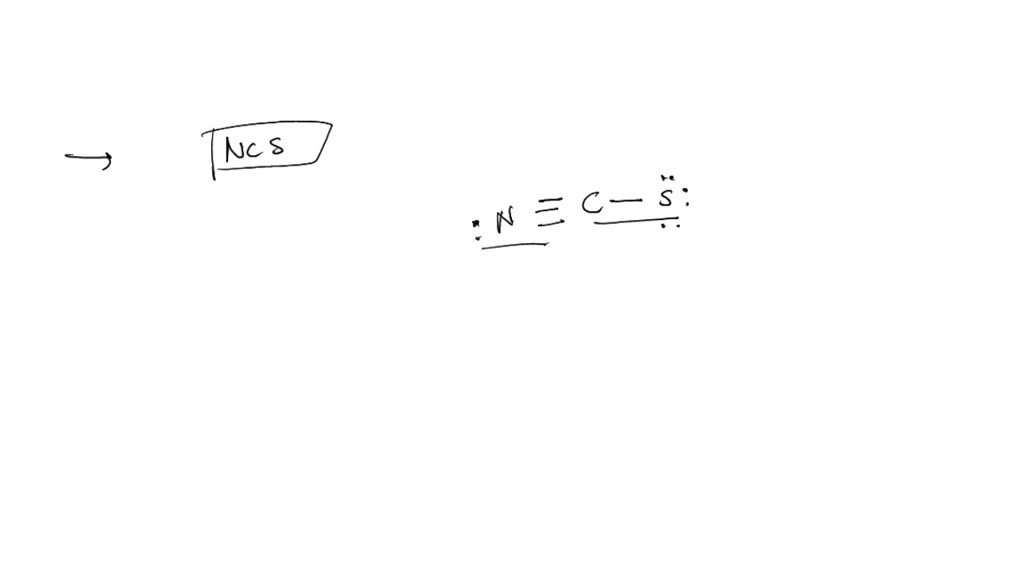
A Lewis Structure For Ncs Is Shown Below However Its Formal Charges **task:** complete the correct lewis structure for ncs⁻ with minimized formal charges based on the structure provided. use the principles of formal charge calculation to adjust the electron distribution accordingly. Using formal charge to predict molecular structure. the arrangement of atoms in a molecule or ion is called its molecular structure.in many cases, following the steps for writing lewis structures may lead to more than one possible molecular structure—different multiple bond and lone pair electron placements or different arrangements of atoms, for instance.
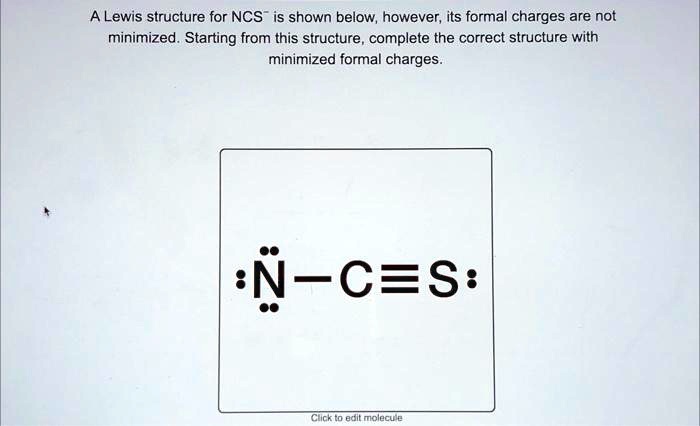
Solved A Lewis Structure For Ncs Is Shown Below However Its Formal
