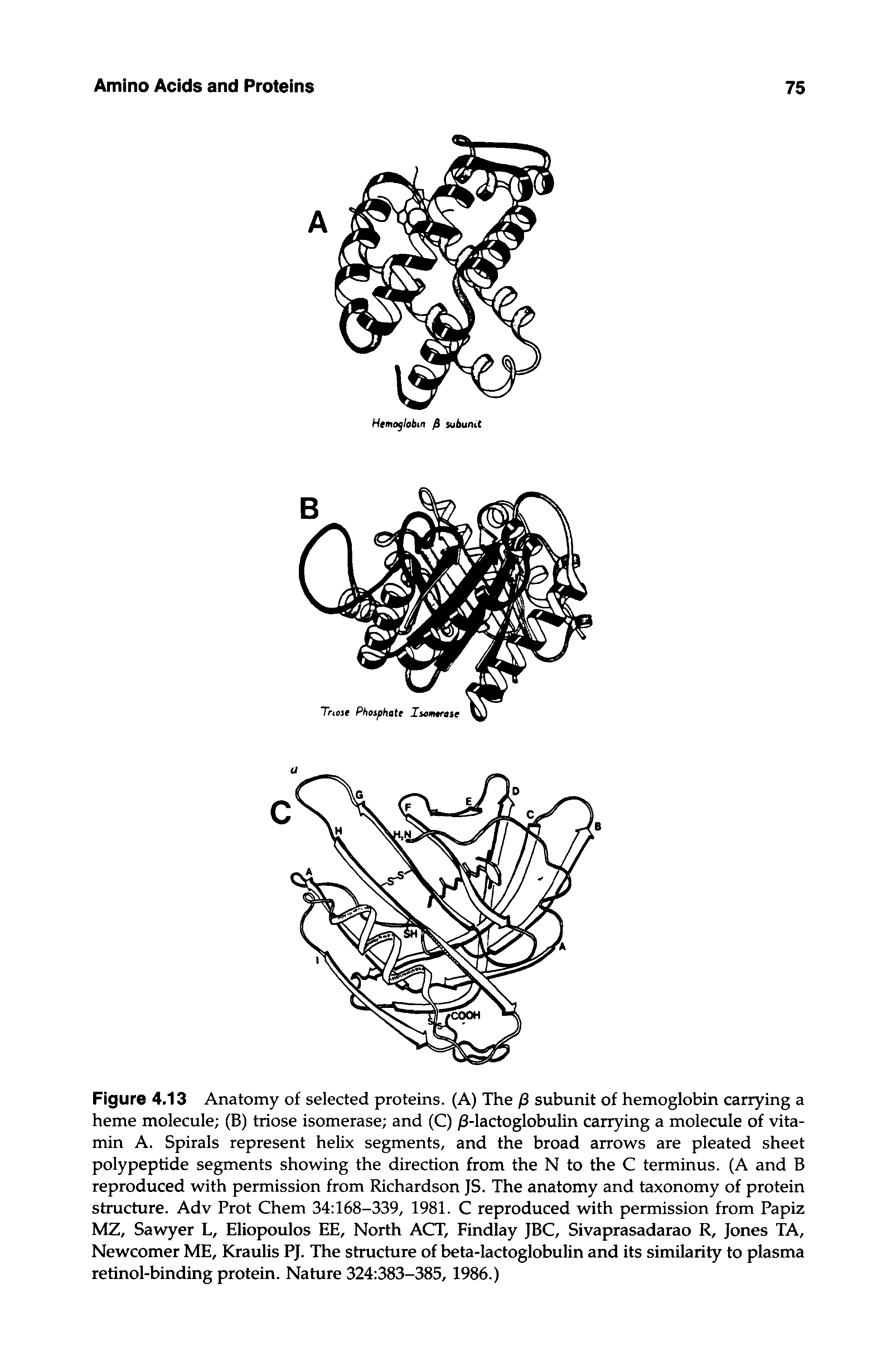
Retinol Binding Protein Structure Big Chemical Encyclopedia Protein protein binding free energy was determined using foldx and the variant number 316 with mutations at 29, 31, 33 positions showed increased binding affinity to ctla 4 ( 21.43. Structure of retinol binding protein showing the position of the four tryptophan residues and retinol molecule in the holo protein. the figure was prepared using the program bobscript (esnouf 1997). the lipocalins are particularly interesting because of their wide range of functions and high levels of sequence divergence with closely similar folds.

Structure Of Retinol Binding Protein Showing The Position Of The Four We identified “intron positions” as amino acid residues on which or just after which introns are found in their corresponding nucleotide sequences, and then found that four intron positions form a plane. we also found that the four intron positions of retinol binding protein encloses its ligand retinol. The complex of retinol with its carrier protein, retinol binding protein (rbp) has been crystallized and its three dimensional structure determined using x ray crystallography. its most striking feature is an eight stranded up and down beta barrel core that completely encapsulates the retinol molecule. This retinol is able to circulate throughout without appreciable loss because it is tightly bound to the 21 000 da plasma retinol binding protein (rbp), first described by goodman and colleagues in 1968 [1]. rbp is synthesized primarily in the liver, where it requires the binding of retinol to trigger its secretion [2]. We report here the crystallographic structure at 3.2 Å of the protein−protein complex of human rbp and ttr. rbp binds at a 2 fold axis of symmetry in the ttr tetramer, and consequently the recognition site itself has 2 fold symmetry: four ttr amino acids (arg 21, val 20, leu 82, and ile 84) are contributed by two monomers.

Structure Of Retinol Binding Protein Showing The Position Of The Four This retinol is able to circulate throughout without appreciable loss because it is tightly bound to the 21 000 da plasma retinol binding protein (rbp), first described by goodman and colleagues in 1968 [1]. rbp is synthesized primarily in the liver, where it requires the binding of retinol to trigger its secretion [2]. We report here the crystallographic structure at 3.2 Å of the protein−protein complex of human rbp and ttr. rbp binds at a 2 fold axis of symmetry in the ttr tetramer, and consequently the recognition site itself has 2 fold symmetry: four ttr amino acids (arg 21, val 20, leu 82, and ile 84) are contributed by two monomers. This letter highlights our efforts in discovering the first, to our knowledge, non retinoid small molecules that bind to rbp4 at the retinol site and reduce serum rbp4 levels in mice, by disrupting the interaction between rbp4 and transthyretin (ttr), a plasma protein that binds rbp4 and protects it from renal excretion. After extracellular binding of rbp4 to stra6, the receptor mediates retinol transport through the membrane and intracellular binding to cellular retinol binding protein 1 (crbp1). subsequent esterification of retinol and storage of retinyl esters, which frees crbp1 to potentially accept another molecule of retinol, is induced by the activity of. Retinol binding protein (rbp) transports vitamin a in the plasma. it consists of eight anti parallel beta strands (a to h) that fold to form an orthogonal barrel. the loops connecting the strands a and b, c and d, and e and f form the entrance to the binding site in the barrel. the retinol molecule is found deep inside this barrel. Four cellular retinol binding protein (crbp) types (crbp1,2,3,4) are encoded in the human genome. here, we report on x ray analyses of human apo and holo crbp1, showing nearly identical structures, at variance with the results of a recent study on the same proteins containing a his tag, which appea ….

Structure Of Retinol Binding Protein Showing The Position Of The Four This letter highlights our efforts in discovering the first, to our knowledge, non retinoid small molecules that bind to rbp4 at the retinol site and reduce serum rbp4 levels in mice, by disrupting the interaction between rbp4 and transthyretin (ttr), a plasma protein that binds rbp4 and protects it from renal excretion. After extracellular binding of rbp4 to stra6, the receptor mediates retinol transport through the membrane and intracellular binding to cellular retinol binding protein 1 (crbp1). subsequent esterification of retinol and storage of retinyl esters, which frees crbp1 to potentially accept another molecule of retinol, is induced by the activity of. Retinol binding protein (rbp) transports vitamin a in the plasma. it consists of eight anti parallel beta strands (a to h) that fold to form an orthogonal barrel. the loops connecting the strands a and b, c and d, and e and f form the entrance to the binding site in the barrel. the retinol molecule is found deep inside this barrel. Four cellular retinol binding protein (crbp) types (crbp1,2,3,4) are encoded in the human genome. here, we report on x ray analyses of human apo and holo crbp1, showing nearly identical structures, at variance with the results of a recent study on the same proteins containing a his tag, which appea ….
