
Crystallization Of A Supersaturated Sucrose Solution Science Some supersaturated solutions spontaneously crystallize when disturbed. more often, adding a seed crystal to a supersaturated solution induces crystallization. keep in mind, crystallization only reduces the concentration of the solution to the point where it is at equilibrium. this is a saturated solution. What it shows: a supersaturated solution is unstable, and by seeding it you can trigger rapid crystallization. how it works: sodium acetate can dissolve in water in great quantities at high temperature, and if you let the solution cool carefully to around room temperature, you have a clear supersaturated solution.

Crystallization Of A Supersaturated Sucrose Solution Science A supersaturated solution definition is given as the one, which contains more dissolved solute than needed for preparing a saturated solution and is prepared by heating a saturated solution, adding excess solute, and then by gently cooling it. A supersaturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature. the recrystallization of the excess dissolved solute in a supersaturated solution can be initiated by the addition of a tiny crystal of solute, called a seed crystal. Reactive crystallization is associated with elevated levels of supersaturation for the crystalline compounds formed by the chemical reaction. in this work, the authors investigated the effect of solvents and supersaturation on polymorphism in vortioxetine hydrobromide prepared by reactive crystallization. Supersaturated sucrose solutions that have been sufficiently cooled without nucleation represent a metastable system in which agitation promotes fast crystalliz.
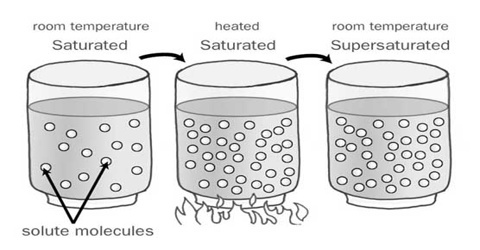
Supersaturated Solution Qs Study Reactive crystallization is associated with elevated levels of supersaturation for the crystalline compounds formed by the chemical reaction. in this work, the authors investigated the effect of solvents and supersaturation on polymorphism in vortioxetine hydrobromide prepared by reactive crystallization. Supersaturated sucrose solutions that have been sufficiently cooled without nucleation represent a metastable system in which agitation promotes fast crystalliz. Exothermic crystallization from a supersaturated solution is demonstrated by pouring a solution of sodium acetate trihydrate onto crystals in a beaker resulting in a column of solid that can be several inches in height. purpose goal: reinforces the concepts of what a solution is composed of, supersaturation and crystallization. Herein, an electron beam induced crystallization method was carried out in in situ liquid cell transmission electron microscopy (tem) to visualize the crystallization of nacl under supersaturated condition in real time. crucial steps and behaviors in the crystallization of nacl were captured and clarified, including the growth of nacl. What is supersaturated solution? a supersaturated solution contains more dissolved solute than required for preparing a saturated solution and can be prepared by heating a saturated solution, adding more solute, and then cooling it gently. excess dissolved solute crystallizes by seeding supersaturated solution with a few crystals of the solute. Under some circumstances it is possible to prepare a solution which behaves anomalously and contains more solute than a saturated solution. such a solution is said to be supersaturated. a good example of supersaturation is provided by na 2 s 2 o 3, sodium thiosulfate, whose solubility at 25°c is 50 g na 2 s 2 o 3 per 100 g h 2 o.
