
Biomarker Testing In Advanced Non Small Cell Lung Cancer More than half of patients with advanced non small cell lung cancer (nsclc) have tumors that harbor an actionable biomarker. early, broad based biomarker testing is essential for determining optimal treatment for nsclc and is recommended by guidelines. Updates to the nccn guidelines for non–small cell lung cancer (nsclc) for 2021 include recommendations for biomarker testing in all appropriate patients with newly diagnosed advanced lung cancer, including squamous cell lung cancer.
Biomarker Testing In Advanced Non Small Cell Lung Cancer Biomarker testing is necessary for determining the optimal treatment of patients newly diagnosed with nsclc. practical guidelines such as the cap iaslc amp, asco, and national comprehensive cancer network guidelines are helpful for determining the most appropriate biomarkers and assays to use. In this review, we discuss the relevant biomarkers used in the clinical management of lung tumors, from diagnosis to prognosis. we also discuss promising new biomarkers, focusing on non small cell lung cancer as the most abundant type of lung cancer. Clinical impact of adherence to nccn guidelines for biomarker testing and first line treatment in advanced non small cell lung cancer (ansclc) using real world electronic health record data. Ve biomarkers will have an impact on a patient’s first line of treatment selection. while lung cancer has paved the w. y, biomarker testing is becoming applicable to multiple other disease states as well. a comparable diagnosis state would be advanced breast cancer, .

Optimizing Immunotherapy Biomarker Testing Vital To Progress In Lung Clinical impact of adherence to nccn guidelines for biomarker testing and first line treatment in advanced non small cell lung cancer (ansclc) using real world electronic health record data. Ve biomarkers will have an impact on a patient’s first line of treatment selection. while lung cancer has paved the w. y, biomarker testing is becoming applicable to multiple other disease states as well. a comparable diagnosis state would be advanced breast cancer, . Numerous trials have led to approval of therapies with corresponding biomarker diagnostics. with so many new treatment options, biomarker testing at diagnosis is important to determine the best options. To fill this gap, this study was designed to compare overall survival (os) among patients who did not receive biomarker testing versus those who did receive biomarker testing (for egfr, braf, ros1, and alk) at 2 different timepoints. In patients with advanced non small cell lung cancer (nsclc), biomarker testing can inform selection of effective targeted therapies as well as avoid therapies that are less likely to be effective in certain populations. Biomarker testing is recommended for all patients diagnosed with non–small cell lung cancer. at a minimum, testing should include the mutations fusions egfr, alk, ros1, and the protein programmed death ligand 1 (pd l1), because fda approved therapies are available for these alterations.

Importance Of Biomarker Testing In Non Small Cell Lung Cancer Numerous trials have led to approval of therapies with corresponding biomarker diagnostics. with so many new treatment options, biomarker testing at diagnosis is important to determine the best options. To fill this gap, this study was designed to compare overall survival (os) among patients who did not receive biomarker testing versus those who did receive biomarker testing (for egfr, braf, ros1, and alk) at 2 different timepoints. In patients with advanced non small cell lung cancer (nsclc), biomarker testing can inform selection of effective targeted therapies as well as avoid therapies that are less likely to be effective in certain populations. Biomarker testing is recommended for all patients diagnosed with non–small cell lung cancer. at a minimum, testing should include the mutations fusions egfr, alk, ros1, and the protein programmed death ligand 1 (pd l1), because fda approved therapies are available for these alterations.
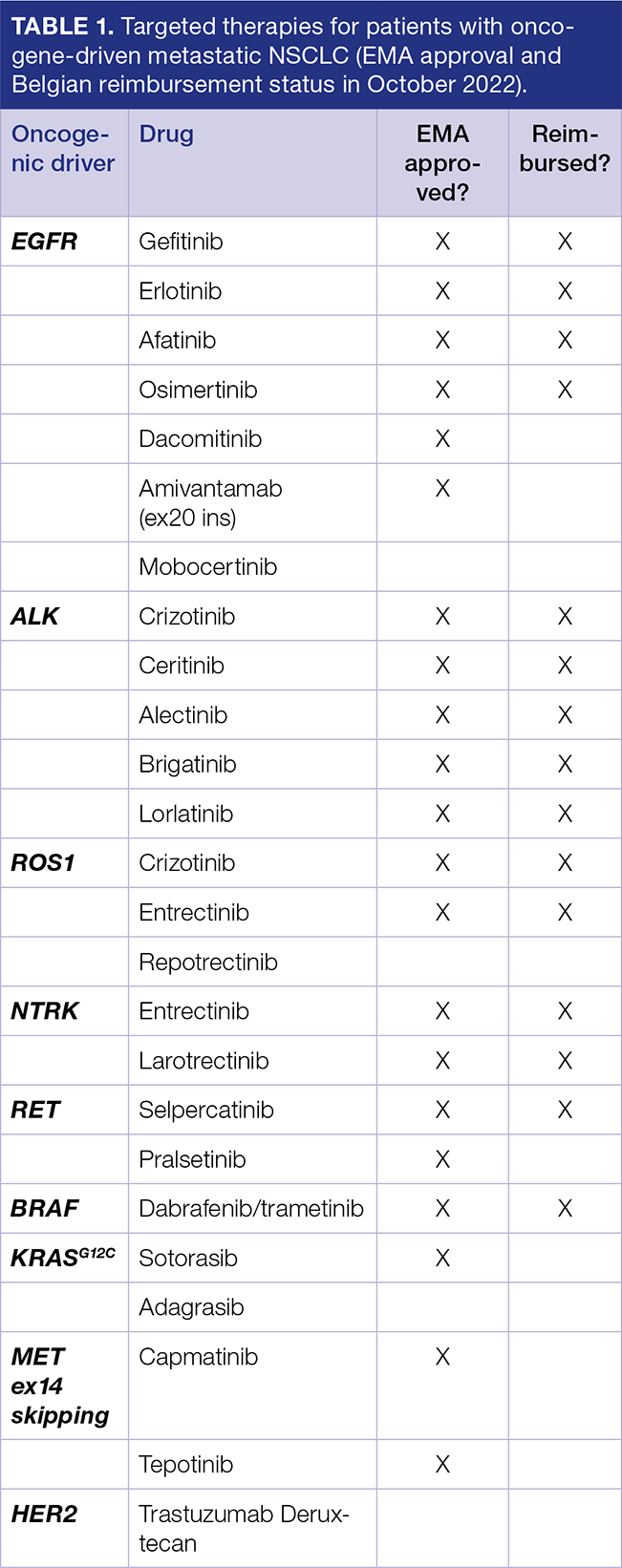
Contemporary Biomarker Testing For Non Small Cell Lung Cancer Bjmo In patients with advanced non small cell lung cancer (nsclc), biomarker testing can inform selection of effective targeted therapies as well as avoid therapies that are less likely to be effective in certain populations. Biomarker testing is recommended for all patients diagnosed with non–small cell lung cancer. at a minimum, testing should include the mutations fusions egfr, alk, ros1, and the protein programmed death ligand 1 (pd l1), because fda approved therapies are available for these alterations.
