The Introduction And Synthesis Route Of Small Molecule Kinase Inhibitors Approved By Fda In 2017

The Introduction And Synthesis Route Of Small Molecule Kinase A total of seven new small molecule kinase inhibitors have been approved by fda in 2017. protein kinases form a very large family of signaling proteins, with more than 500 encoded in the human genome. In this minireview we discuss the most common therapeutic indications and molecular target (s) of kinase inhibitors fda approved 2018–2023.
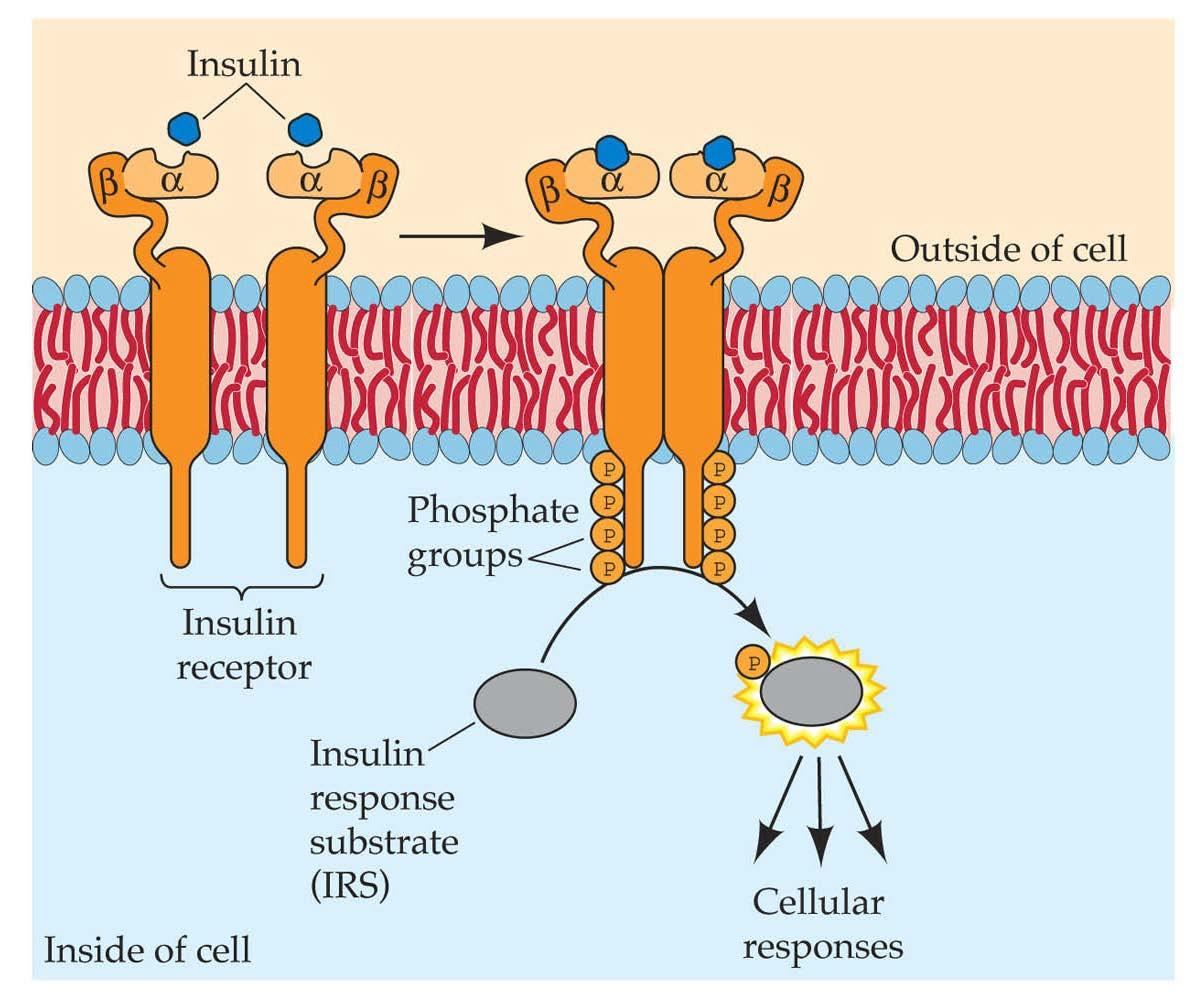
Approved Small Molecule Kinase Non Oncology Inhibitors Oncology This review summarizes the physicochemical properties of all 80 fda approved small molecule protein kinase inhibitors including the molecular weight, number of hydrogen bond donors acceptors, polar surface area, potency, solubility, lipophilic efficiency, and ligand efficiency. Along with the therapeutic and pharmacological properties of each kinase inhibitor approved across the world until 2020, we provide the synthesis routes originally used during the. Here, we assess the key structural and physicochemical properties, target selectivity and mechanism of function, and therapeutic indications of these approved inhibitors. Along with the therapeutic and pharmacological properties of each kinase inhibitor approved across the world until 2020, we provide the synthesis routes originally used during the discovery phase, many of which were only available in patent applications.

Small Molecule Kinase Inhibitors Approved By Fda In 2021 Here, we assess the key structural and physicochemical properties, target selectivity and mechanism of function, and therapeutic indications of these approved inhibitors. Along with the therapeutic and pharmacological properties of each kinase inhibitor approved across the world until 2020, we provide the synthesis routes originally used during the discovery phase, many of which were only available in patent applications. Along with the therapeutic and pharmacological properties of each kinase inhibitor approved across the world until 2020, we provide the synthesis routes originally used during the discovery phase, many of which were only available in patent applications. With the present special issue entitled: "design and synthesis of small molecule kinase inhibitors", we would like to encourage the submission of original research articles and reviews in the field, highlighting the role of novel kinases inhibitors as promising treatments in cancer. Herein, we first provide an updated overview of the approved smkis, with an emphasis on their binding modes, classi ed in groups of type fi i and ii atp competitive inhibitors, type iii and iv allosteric inhibitors, and covalent inhibitors. Here we present all approved small molecule kinase inhibitors with an emphasis on binding mechanism and structural features, summarize current challenges, and discuss future directions in this field.

Us Fda Approved Small Molecule Therapeutic Protein Kinase Inhibitors Along with the therapeutic and pharmacological properties of each kinase inhibitor approved across the world until 2020, we provide the synthesis routes originally used during the discovery phase, many of which were only available in patent applications. With the present special issue entitled: "design and synthesis of small molecule kinase inhibitors", we would like to encourage the submission of original research articles and reviews in the field, highlighting the role of novel kinases inhibitors as promising treatments in cancer. Herein, we first provide an updated overview of the approved smkis, with an emphasis on their binding modes, classi ed in groups of type fi i and ii atp competitive inhibitors, type iii and iv allosteric inhibitors, and covalent inhibitors. Here we present all approved small molecule kinase inhibitors with an emphasis on binding mechanism and structural features, summarize current challenges, and discuss future directions in this field.

Us Fda Approved Small Molecule Therapeutic Protein Kinase Inhibitors Herein, we first provide an updated overview of the approved smkis, with an emphasis on their binding modes, classi ed in groups of type fi i and ii atp competitive inhibitors, type iii and iv allosteric inhibitors, and covalent inhibitors. Here we present all approved small molecule kinase inhibitors with an emphasis on binding mechanism and structural features, summarize current challenges, and discuss future directions in this field.

Us Fda Approved Small Molecule Therapeutic Protein Kinase Inhibitors
Comments are closed.