Understanding Atom Structure Nucleus Proton Electron Neutron
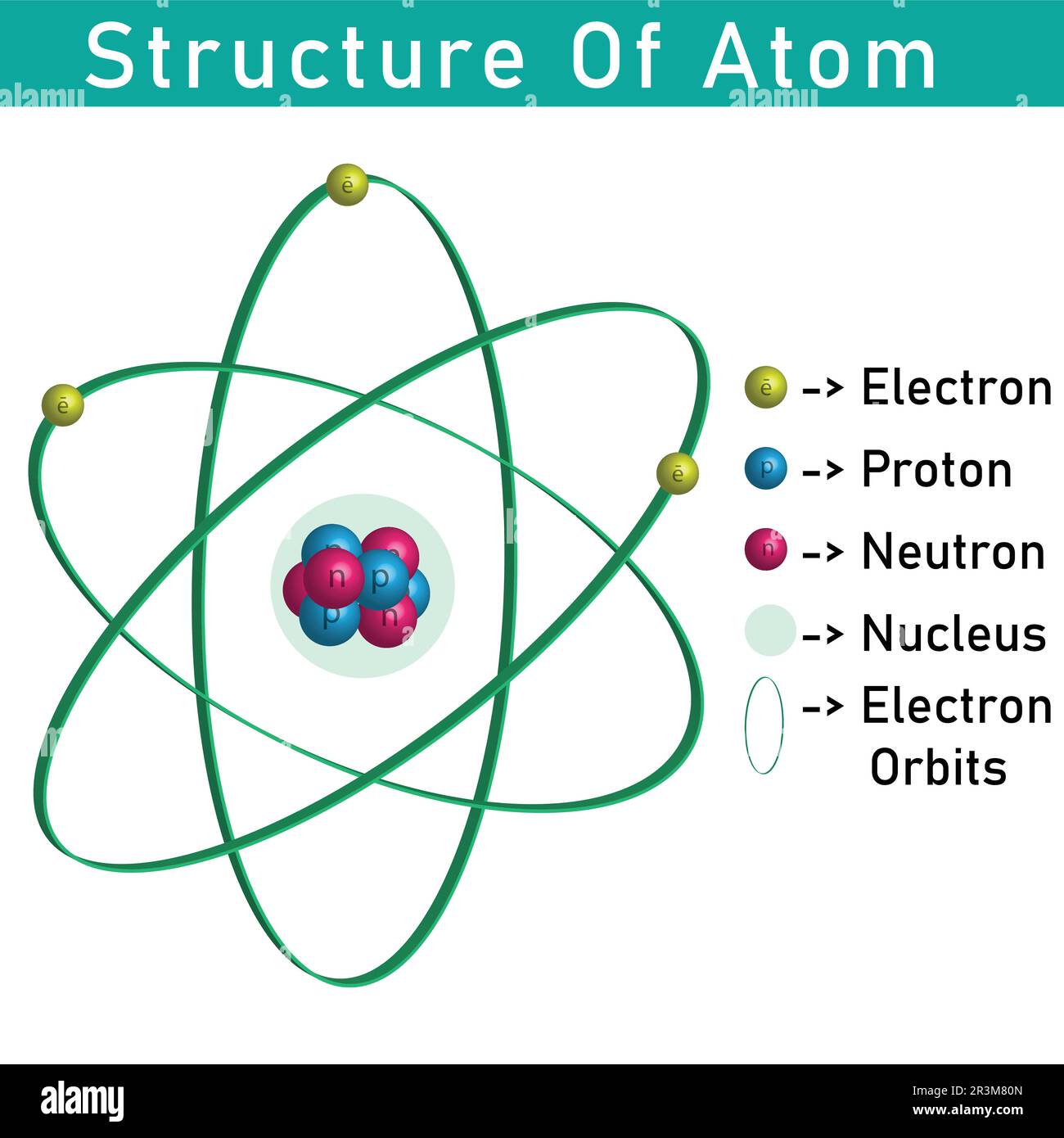
3d Structure Of Atom Showing Nucleus Proton Neutron And Electron Subatomic particles an atom is the smallest particle of an element that represents its properties. it comprises subatomic particles called electrons, protons, and neutrons. electrons (e ) carry a negative charge equal to the unit elementary charge (\ (e\)), i.e., 1 \ (e\), and have a mass equal to 9.11×10−31 kg. protons (p ) carry a positive charge equal to 1\ (e\) and a mass of 1.6726×. The center of an atom is called the nucleus and is made up of both protons and neutrons. this part of the atom is able to determine a wide range of properties, such as the atomic number and atomic mass.
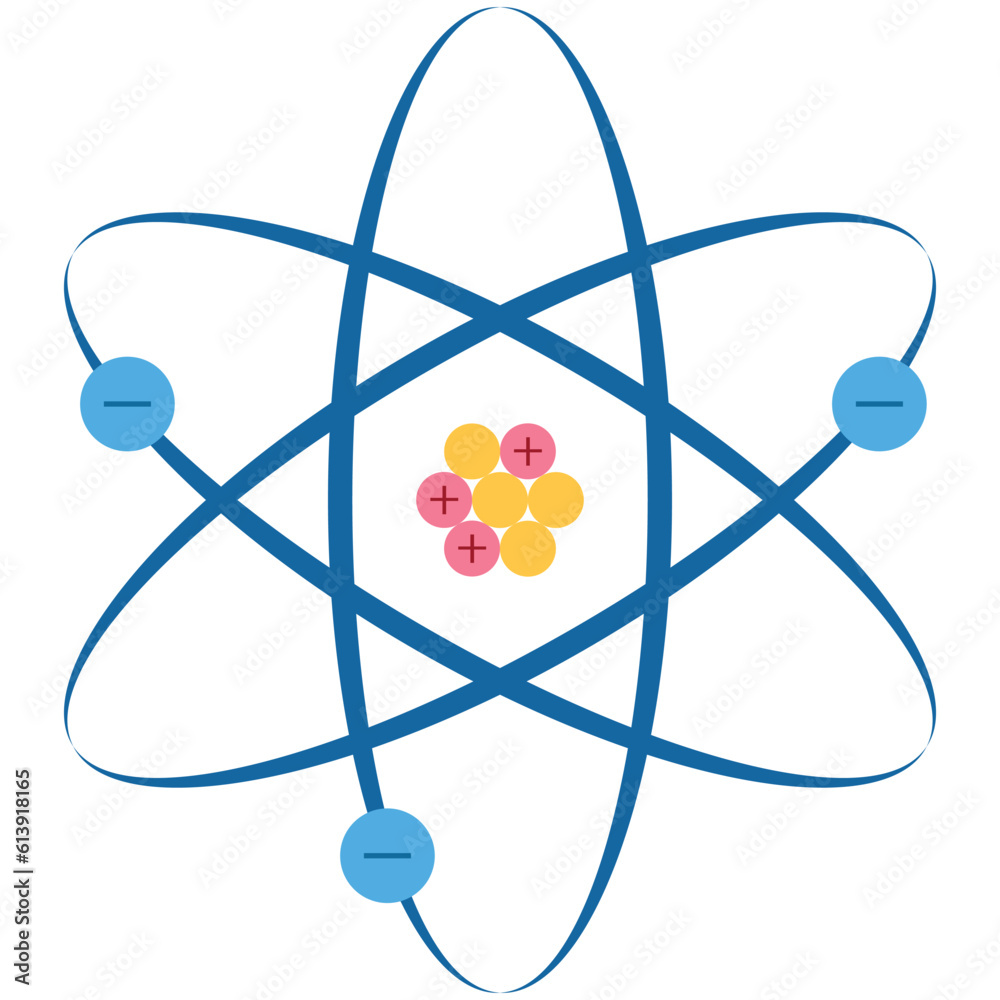
Model Of The Atom Proton Neutron Electron And Nucleus The Atomic Though atoms are incredibly small—measured in nanometers—they are made up of even smaller subatomic particles: protons, neutrons, and electrons. understanding how these particles are arranged and how they interact is essential to understanding chemical reactions, elements, and the periodic table. Protons (positively charged) and neutrons (neutral) are found in the nucleus at the centre of the atom. electrons (negatively charged) orbit the nucleus in shells (energy levels) the first electron shell holds up to two electrons; the second shell holds up to eight electrons. The atom’s nucleus holds the protons and neutrons, whereas the electron’s orbit lies in discrete energy levels. the electron is inversely charged, the neutron is neutral, and the proton is positively charged. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. discuss the electronic and structural properties of an atom.

Components Of Atom Proton Electron Neutron And Nucleus Vector The atom’s nucleus holds the protons and neutrons, whereas the electron’s orbit lies in discrete energy levels. the electron is inversely charged, the neutron is neutral, and the proton is positively charged. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. discuss the electronic and structural properties of an atom. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. the numbers of subatomic particles in an atom can be calculated from its atomic number and. Within the nucleus, protons and neutrons are packed tightly together, with protons carrying a positive charge and neutrons being electrically neutral. electrons, on the other hand, orbit the nucleus in distinct energy levels, each with a negative charge. Neutrons and protons make up the nucleus of an atom, while electrons circle this nucleus. the number of these particles that make up an atom are what help differentiate elements from one another, with elements containing more protons listed higher on the periodic chart. This diagram offers a way to visualize how atoms are put together, showing the nucleus where protons and neutrons reside, and the electron shells that surround the nucleus.
Comments are closed.