
Understanding The Effect Of Ph On The Solubility Of Ionic Compo Pdf The rule you need, then, is that the solubility of any ionic compound containing an ion that acts as a bronstedlowry base is higher in acidic (low ) conditions. let's apply this rule to each compound in the table. To develop an understanding as to how the relative acidity or basicity of a drug substance is able to influence its aqueous solubility, the principles of chemical equilibria as applied to weakly ionic substances will first be developed.

Solved Understanding The Effect Of Ph On The Solubility Of Chegg The apparent solubility of a monoacid or a monobase is the sum of its intrinsic solubility so (solubility in its molecular state) and its ionic maximum concentration (solubility in its ionic state). the latter is a function of the ph value, a function that is easily stated. let’s consider an acid ha that is poorly soluble in its molecular. We propose a new way to understand these equilibria by implementing a method in which all conditions appear naturally in the mathematical equations. we present the excess parameter with. Solubility equilibria what we will learn: • homogeneous and heterogeneous solution equilibria • common ion effect • buffer solutions • acid base titrations • acid base indicators • solubility equilibria • separation of ions by fractional precipitation • common ion effect and solubility • ph and solubility. The state of ionization of a substance will often profoundly affect its degree of aqueous solubility, as evidenced by the high solubility of sodium benzoate as opposed to the low solubility.
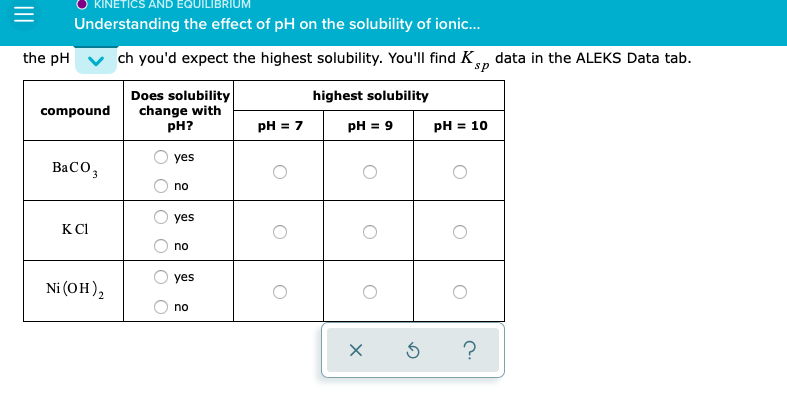
Solved Understanding The Effect Of Ph On The Solubility Of Chegg Solubility equilibria what we will learn: • homogeneous and heterogeneous solution equilibria • common ion effect • buffer solutions • acid base titrations • acid base indicators • solubility equilibria • separation of ions by fractional precipitation • common ion effect and solubility • ph and solubility. The state of ionization of a substance will often profoundly affect its degree of aqueous solubility, as evidenced by the high solubility of sodium benzoate as opposed to the low solubility. The solubility of a drug is defined as the maximum quantity of a drug dissolved in a given volume of a solvent at chosen temperature, pressure and ph. for ionizable drugs, the solubility can be affected by the ph of the solution, and the intrinsic solubility (s 0) is defined as the concentration of. To solve this problem, you must ask yourself how changing the (i.e. the concentration of. ) might affect the dissolution equilibrium of each compound. the answer is that if one of the product ions acts as a brønstedlowry base, it will react with any excess and be removed from solution. Question: understanding the effect of ph on the solubility of ionic compo for each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. In this section, we discuss the relationship between the solubility of these classes of compounds and ph. we begin our discussion by examining the effect of ph on the solubility of a representative salt, m a −, where a − is the conjugate base of the weak acid ha. when the salt dissolves in water, the following reaction occurs:.
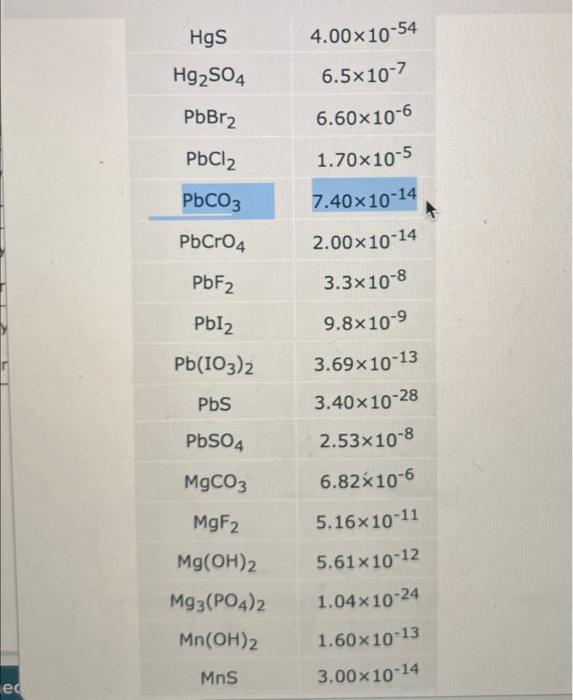
Solved Understanding The Effect Of Ph On The Solubility Of Chegg The solubility of a drug is defined as the maximum quantity of a drug dissolved in a given volume of a solvent at chosen temperature, pressure and ph. for ionizable drugs, the solubility can be affected by the ph of the solution, and the intrinsic solubility (s 0) is defined as the concentration of. To solve this problem, you must ask yourself how changing the (i.e. the concentration of. ) might affect the dissolution equilibrium of each compound. the answer is that if one of the product ions acts as a brønstedlowry base, it will react with any excess and be removed from solution. Question: understanding the effect of ph on the solubility of ionic compo for each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. In this section, we discuss the relationship between the solubility of these classes of compounds and ph. we begin our discussion by examining the effect of ph on the solubility of a representative salt, m a −, where a − is the conjugate base of the weak acid ha. when the salt dissolves in water, the following reaction occurs:.
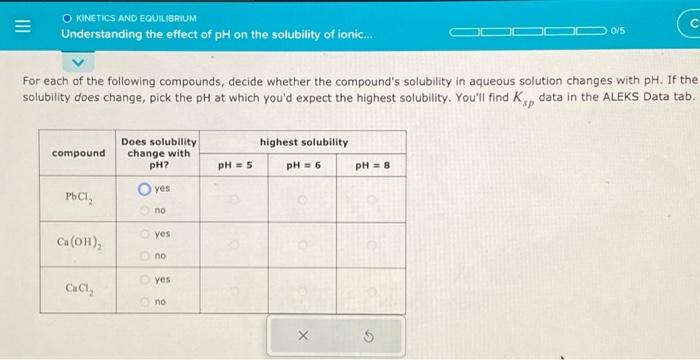
Solved Understanding The Effect Of Ph On The Solubility Of Chegg Question: understanding the effect of ph on the solubility of ionic compo for each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. In this section, we discuss the relationship between the solubility of these classes of compounds and ph. we begin our discussion by examining the effect of ph on the solubility of a representative salt, m a −, where a − is the conjugate base of the weak acid ha. when the salt dissolves in water, the following reaction occurs:.
