Unit C Sction 2 1 Heat Affects Matter

Unit C Sction 2 1 Heat Affects Matter Ppt This document discusses the particle model of matter and how heat affects the states of matter. it explains that matter exists in three states solid, liquid, and gas and can change between these states by adding or removing heat energy. Study with quizlet and memorize flashcards containing terms like phases of matter, solid, liquid and more.
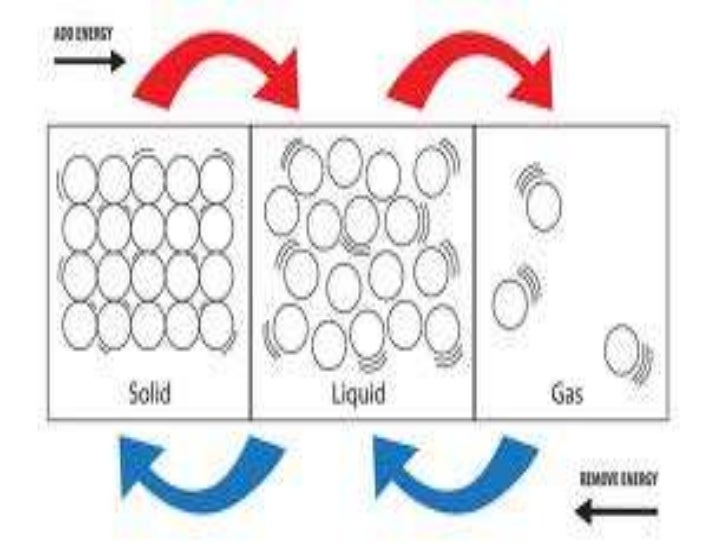
Unit C Sction 2 1 Heat Affects Matter Section 1: theory of heat unit 2: matter and energy unit objectives after studying this chapter, you should be able to: define matter. list the three states in which matter is commonly found. Describe the nature of thermal energy and its effects on different forms of matter, using informal observations, experimental evidence and models. Effects of heat on a substance, i.e., temperature change or change of physical state of the substance are described. This document discusses the three states of matter solid, liquid, and gas providing examples of each.

Unit C Sction 2 1 Heat Affects Matter Ppt Effects of heat on a substance, i.e., temperature change or change of physical state of the substance are described. This document discusses the three states of matter solid, liquid, and gas providing examples of each. Study with quizlet and memorise flashcards containing terms like particle model of matter (1), particle model of matter (2), heat energy and others. Heat is a form of energy that increases the temperature of an object or substance. when heat is applied to any substance, it affects the atoms and molecules that make up that substance. these changes can be physical or chemical, depending on the conditions and materials involved. Particles that make up matter just can't stand still, but they can slow down a bit when they get a little chilly. but what happens when they get hot? let's take a look at what happens to the three different states of matter when exposed to heat, and then we will return to the question. Heat is the energy that transfers from one substance to another because of the difference in kinetic energy. the average energy of the particles the temperature of the substance is affected, by increasing or decreasing.
Comments are closed.