Unit Cell Chemistry Simple Cubic Body Centered Cubic Face Centered Cubic Crystal Lattice Structu
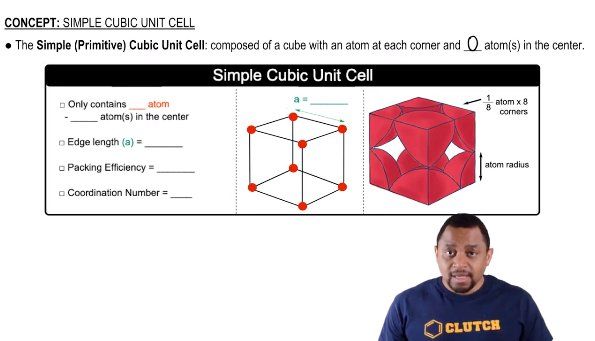
Unit Cell Chemistry Simple Cubic Body Centered Cubic Face Cen This chemistry video tutorial provides a basic introduction into unit cell and crystal lattice structures. it highlights the key differences between the simple cubic unit cell,. In the body centered cubic cell (bcc) there is an additional atom in the center of the cube, and in the face centered cubic cell, an atom is shared between two unit cells along the face.
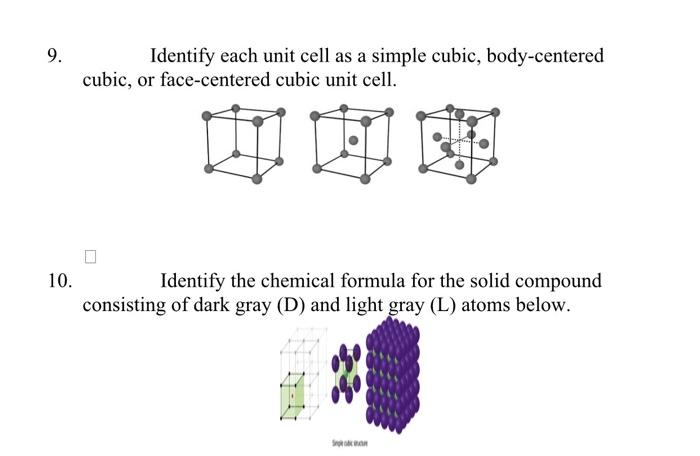
Solved Identify Each Unit Cell As A Simple Cubic Chegg We will focus on the cubic category, which includes the three types of unit cells simple cubic, body centered cubic, and face centered cubic shown in the figure below. Define and determine the coordination numbers for crystalline solids. perform calculations relating the density and edge length for simple cubic, body centered cubic, and face centered cubic unit cells. The body centered cubic (bcc) crystal structure is one of the most common ways for atoms to arrange themselves in metals. the bcc crystal structure is based on the bravais lattice of the same name, with 1 atom per lattice point at each corner of the cube and the center of the cube. Calculation of number of particles per unit cell of a cubic crystal system. keeping the following points in mind we can calculate the number of atoms in a unit cell. an atom at the corner is shared by eight unit cells. hence an atom at the corner contributes 1 8 to the unit cell. contribution of each atom on the face is 1 2 to the unit cell.
Unit Cells Simple Cubic Body Centered Cubic And Face Centered Cubic The body centered cubic (bcc) crystal structure is one of the most common ways for atoms to arrange themselves in metals. the bcc crystal structure is based on the bravais lattice of the same name, with 1 atom per lattice point at each corner of the cube and the center of the cube. Calculation of number of particles per unit cell of a cubic crystal system. keeping the following points in mind we can calculate the number of atoms in a unit cell. an atom at the corner is shared by eight unit cells. hence an atom at the corner contributes 1 8 to the unit cell. contribution of each atom on the face is 1 2 to the unit cell. Study with quizlet and memorize flashcards containing terms like body centered cubic (bcc) solid, body centered cubic unit cell, cubic closest packing (ccp) and more. As one example, the cubic crystal system is composed of three different types of unit cells: (1) simple cubic, (2) face centered cubic, and (3) body centered cubic. Join thousands of students who trust us to help them ace their exams! watch the first video. When we consider a cubic crystal system, the lattice point exists in the center, center of each face, and at the corner of a cube and forms three types of unit cells primitive or simple unit cell, body centered unit cell, and face centered unit cell.

Unit Cells Simple Cubic Body Centered Cubic And Face Centered Cubic Study with quizlet and memorize flashcards containing terms like body centered cubic (bcc) solid, body centered cubic unit cell, cubic closest packing (ccp) and more. As one example, the cubic crystal system is composed of three different types of unit cells: (1) simple cubic, (2) face centered cubic, and (3) body centered cubic. Join thousands of students who trust us to help them ace their exams! watch the first video. When we consider a cubic crystal system, the lattice point exists in the center, center of each face, and at the corner of a cube and forms three types of unit cells primitive or simple unit cell, body centered unit cell, and face centered unit cell.
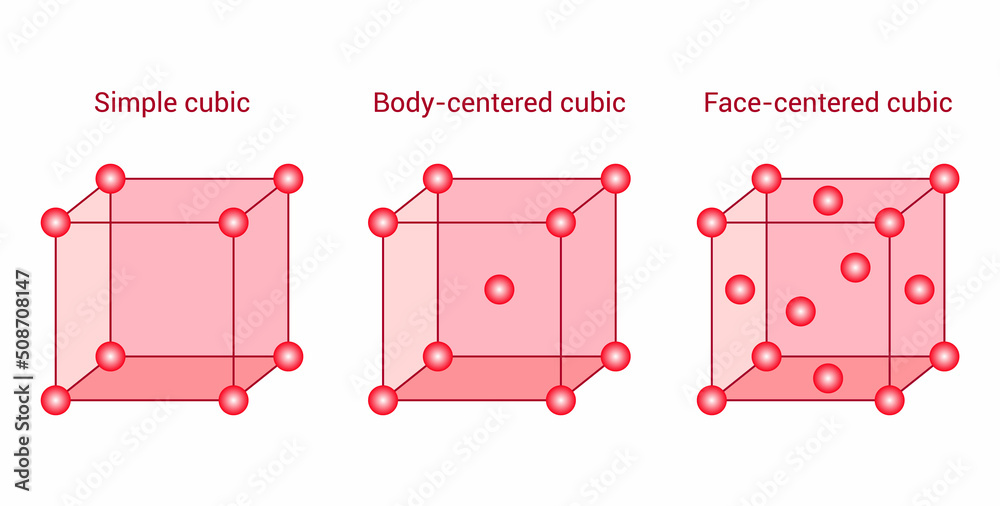
Three Types Of Cubic Unit Cells Simple Cubic Body Centered Cubic And Join thousands of students who trust us to help them ace their exams! watch the first video. When we consider a cubic crystal system, the lattice point exists in the center, center of each face, and at the corner of a cube and forms three types of unit cells primitive or simple unit cell, body centered unit cell, and face centered unit cell.
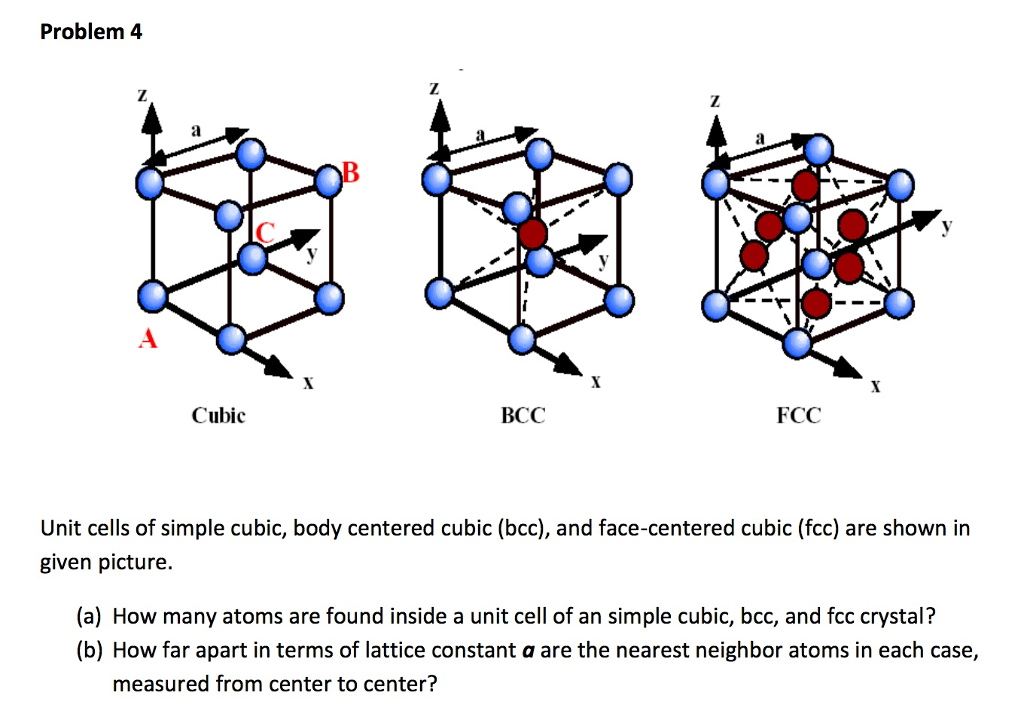
Solved Unit Cells Of Simple Cubic Body Centered Cubic B Chegg
Comments are closed.