Using The Solubility Of A Compound To Calculate Ksp4 Aleks Jimmy

Solved Kinetics And Equilibrium Using Ksp To Calculate The Chegg This video is designed to help students working on aleks chemistry homework. it covers how to calculate the solubility in both moles l and g l when given the ksp using an ice table. Learn how to use the solubility of a compound to calculate ksp, and see examples that walk through sample problems step by step for you to improve your chemistry knowledge and.
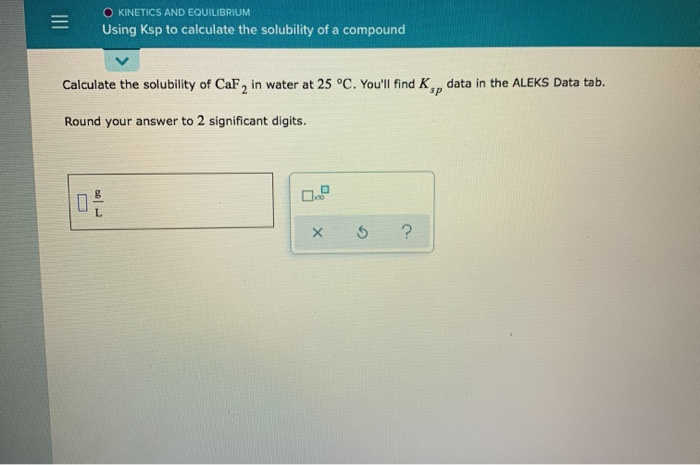
Solved Kinetics And Equilibrium Using Ksp To Calculate The Chegg To use this equation, you first need to convert the equilibrium solubilityof to the equilibrium molar solubility. dividing grams per liter by the molar mass of will convert to moles per liter: keep a few extra digits for now. Given this value, how does one go about calculating the k sp of the substance? here is a skeleton outline of the process: 1) write the chemical equation for the substance dissolving and dissociating. 2) write the k sp expression. 3) insert the concentration of each ion and multiply out. Calculate the solubility of cubr in water at 25∘c. you'll find ksp data in the alfks data tab. hound your answer to 2 significant digits:. Solubility data can be used to calculate the ksp for a given compound. the following steps need to be taken. convert from solubility to molar solubility. use the dissociation equation to determine the concentration of each of the ions in mol l. apply the ksp equation. sample problem: calculating ksp from solubility.
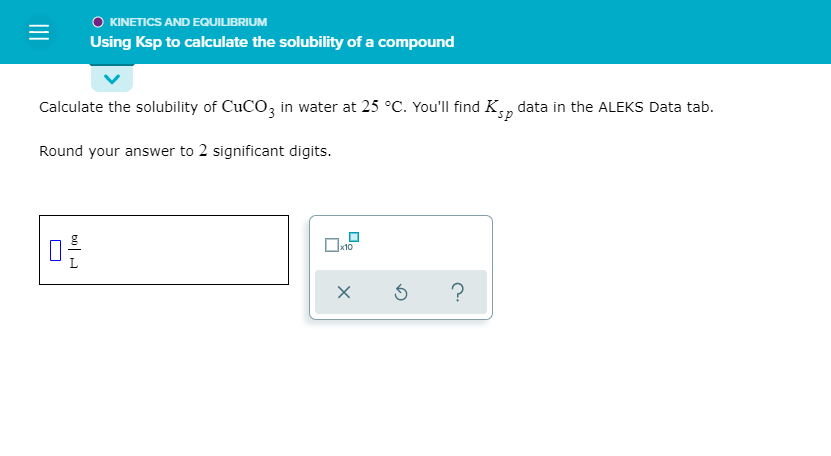
Solved Kinetics And Equilibrium Using Ksp To Calculate The Chegg Calculate the solubility of cubr in water at 25∘c. you'll find ksp data in the alfks data tab. hound your answer to 2 significant digits:. Solubility data can be used to calculate the ksp for a given compound. the following steps need to be taken. convert from solubility to molar solubility. use the dissociation equation to determine the concentration of each of the ions in mol l. apply the ksp equation. sample problem: calculating ksp from solubility. Aleks: using the solubility of a compound to calculate ksp roxi hulet 28.8k subscribers subscribed. By using the solubility product constant (ksp) calculator, you can input the ion concentrations to determine if the solution exceeds the ksp value, indicating a potential precipitate formation. this helps in predicting the outcome of the reaction accurately. In a saturated solution, the last bit of undissolved will be in equilibrium with the dissolved ions : that means you can calculate the equilibrium molarities of the ions from the expression equation. doesn't appear here because it's present as a pure solid. Learn how to use ksp to calculate the solubility of a compound, and see examples that walk through sample problems step by step for you to improve your chemistry knowledge and.
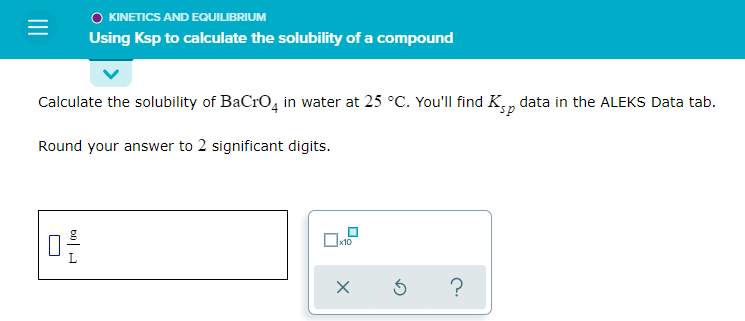
Solved Kinetics And Equilibrium Using Ksp To Calculate The Chegg Aleks: using the solubility of a compound to calculate ksp roxi hulet 28.8k subscribers subscribed. By using the solubility product constant (ksp) calculator, you can input the ion concentrations to determine if the solution exceeds the ksp value, indicating a potential precipitate formation. this helps in predicting the outcome of the reaction accurately. In a saturated solution, the last bit of undissolved will be in equilibrium with the dissolved ions : that means you can calculate the equilibrium molarities of the ions from the expression equation. doesn't appear here because it's present as a pure solid. Learn how to use ksp to calculate the solubility of a compound, and see examples that walk through sample problems step by step for you to improve your chemistry knowledge and.
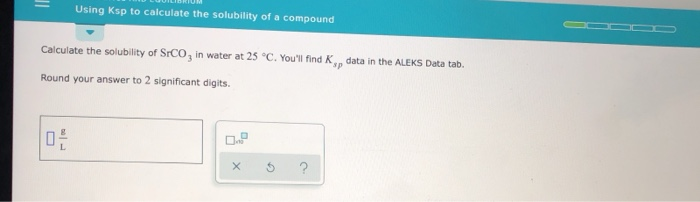
Using Ksp To Calculate The Solubility Of A Compound Chegg In a saturated solution, the last bit of undissolved will be in equilibrium with the dissolved ions : that means you can calculate the equilibrium molarities of the ions from the expression equation. doesn't appear here because it's present as a pure solid. Learn how to use ksp to calculate the solubility of a compound, and see examples that walk through sample problems step by step for you to improve your chemistry knowledge and.
Comments are closed.