Using Your Measured Value Of Ksp Calculate The Molar Chegg

Using Your Measured Value Of Ksp Calculate The Molar Chegg Science chemistry chemistry questions and answers using your measured value of ksp, calculate the molar solubility of ca (oh)2 in the following:. The solubility (by which we usually mean the molar solubility) of a solid is expressed as the concentration of the "dissolved solid" in a saturated solution. in the case of a simple 1:1 solid such as agcl, this would just be the concentration of ag or cl – in the saturated solution.
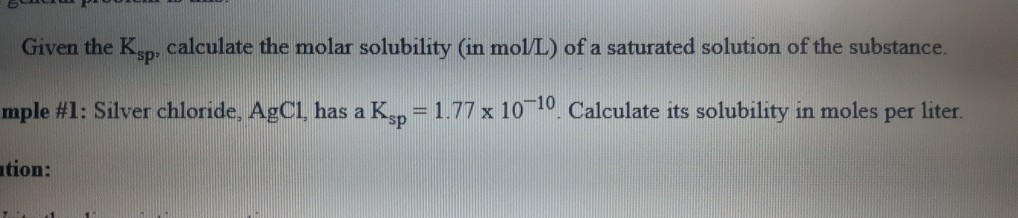
Solved Given The Ksp Calculate The Molar Solubility In Chegg With this information, you can find the molar solubility which is the number of moles that can be dissolved per liter solution until the solution becomes saturated. The **molar solubility **of a compound is the number of moles of the compound that can dissolve per liter of solution before reaching saturation. to calculate the molar solubility, we need to use the equilibrium expression for the dissolution of the compound, as well as the given ksp value. Given this value, how does one go about calculating the k sp of the substance? here is a skeleton outline of the process: 1) write the chemical equation for the substance dissolving and dissociating. 2) write the k sp expression. 3) insert the concentration of each ion and multiply out. To use this calculator, input the values for the solubility product constant (ksp), and the coefficients n and m representing the stoichiometric amounts of cation and anion in the dissociation equation of the salt.
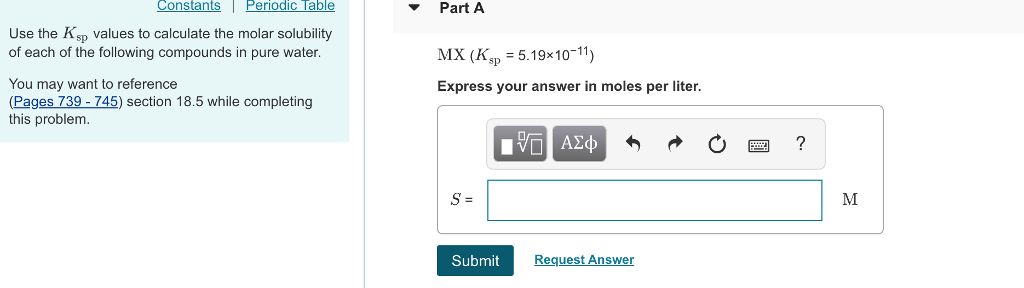
Solved Constants Periodic Table Part A Use The Ksp Values Chegg Given this value, how does one go about calculating the k sp of the substance? here is a skeleton outline of the process: 1) write the chemical equation for the substance dissolving and dissociating. 2) write the k sp expression. 3) insert the concentration of each ion and multiply out. To use this calculator, input the values for the solubility product constant (ksp), and the coefficients n and m representing the stoichiometric amounts of cation and anion in the dissociation equation of the salt. There are 4 steps to solve this one. use the values of ksp given to calculate the molar solubility of the following compounds. Enter the ksp, coefficient of ion 1, and coefficient of ion 2 into the calculator to determine the molar solubility. To find the molar solubility of mg (oh)2, one must relate the concentrations of the ions produced upon dissolution to the ksp value, allowing for the calculation of how many moles of mg (oh)2 can dissolve in water. Which of the following provides the correct comparison of q and ksp, and describes the overall process that occurs at any point in the unshaded region y of the graph?.
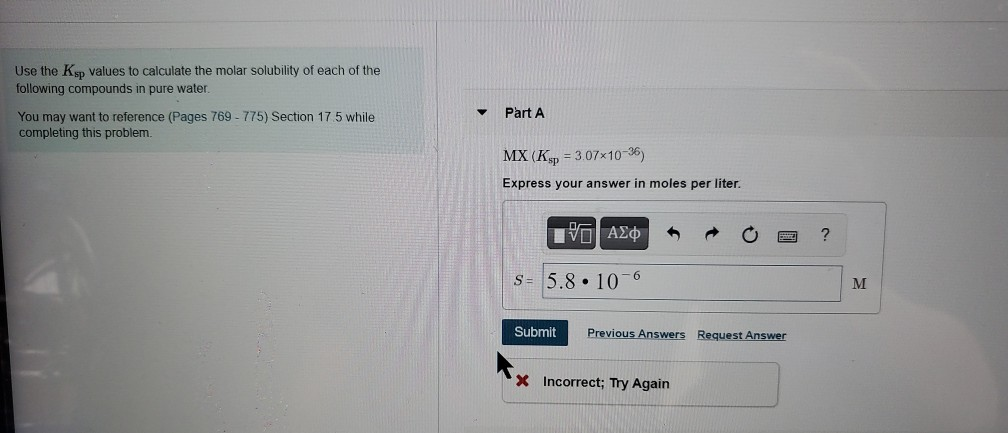
Solved Use The Ksp Values To Calculate The Molar Solubility Chegg There are 4 steps to solve this one. use the values of ksp given to calculate the molar solubility of the following compounds. Enter the ksp, coefficient of ion 1, and coefficient of ion 2 into the calculator to determine the molar solubility. To find the molar solubility of mg (oh)2, one must relate the concentrations of the ions produced upon dissolution to the ksp value, allowing for the calculation of how many moles of mg (oh)2 can dissolve in water. Which of the following provides the correct comparison of q and ksp, and describes the overall process that occurs at any point in the unshaded region y of the graph?.
Comments are closed.