What Are Solubility And Molar Solubility Check All That Apply Group

Solved What Are Solubility And Molar Solubility Check All Chegg In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. insolubility is the opposite property, the inability of the solute to form such a solution. Solubility is the ability of a solute to dissolve in a solvent to form a solution. this is the property that allows things like sugar molecules to dissolve in a cup of coffee.
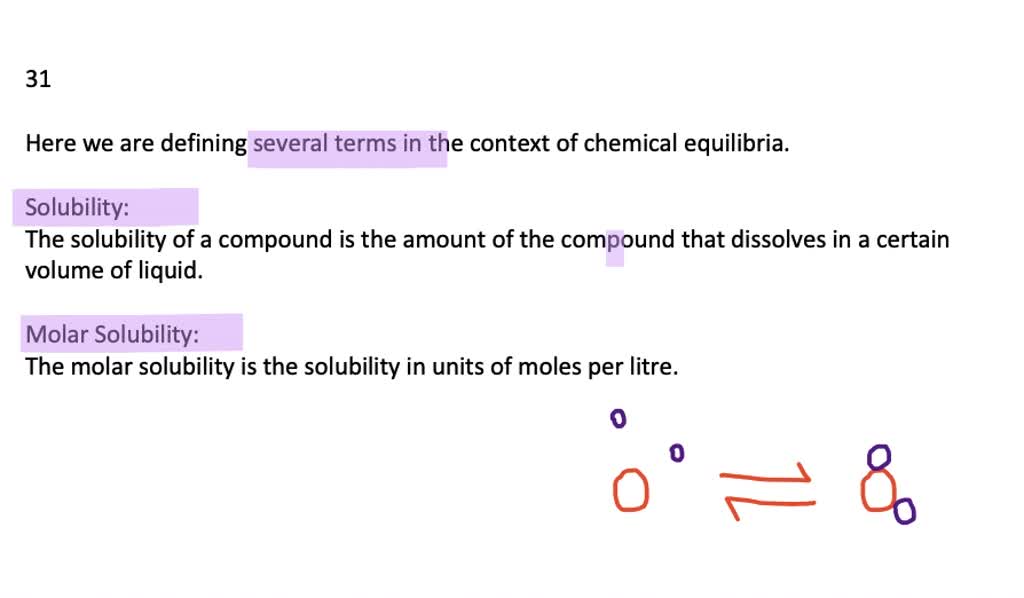
Solved What Are Solubility And Molar Solubility Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium. in such an equilibrium, le chatelier's principle can be used to explain most of the main factors that affect solubility. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. the process through which a solute in its solid, liquid, or gaseous phase dissolves in a solvent to produce a solution is called dissolution. Solubility, degree to which a substance dissolves in a solvent to make a solution (usually expressed as grams of solute per litre of solvent). solubility of one fluid (liquid or gas) in another may be complete (totally miscible; e.g., methanol and water) or partial (oil and water dissolve only. Solubility is a fundamental concept in chemistry that governs how substances interact, forming the basis for countless natural phenomena and industrial processes. it describes the capacity of a substance to mix uniformly with another, creating a homogeneous blend. what solubility really means solubility refers to the ability of a substance, known as the solute, to dissolve in another substance.
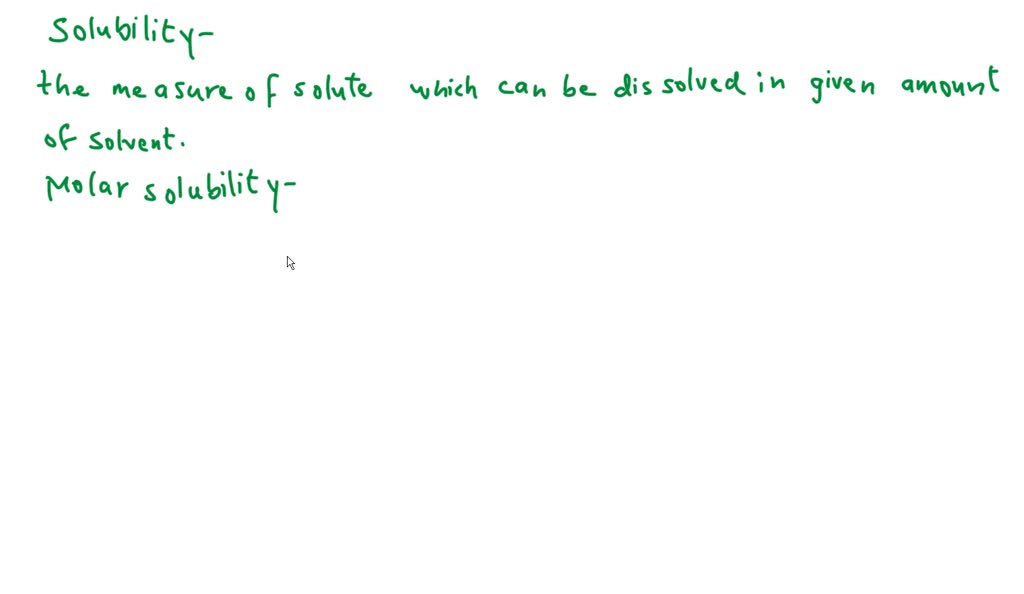
鈴 Olved What Are Solubility And Molar Solubility Numerade Solubility, degree to which a substance dissolves in a solvent to make a solution (usually expressed as grams of solute per litre of solvent). solubility of one fluid (liquid or gas) in another may be complete (totally miscible; e.g., methanol and water) or partial (oil and water dissolve only. Solubility is a fundamental concept in chemistry that governs how substances interact, forming the basis for countless natural phenomena and industrial processes. it describes the capacity of a substance to mix uniformly with another, creating a homogeneous blend. what solubility really means solubility refers to the ability of a substance, known as the solute, to dissolve in another substance. What is solubility? the maximum amount of solute that can dissolve in a known quantity of solvent at a certain temperature is its solubility. a solution is a homogeneous mixture of one or more solutes in a solvent. sugar cubes added to a cup of tea or coffee are a common example of a solution. The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt. solubility rules. there are a number of patterns in the data obtained from measuring the solubility of different salts. Solubility is defined as the maximum quantity of a substance that can be dissolved in another. it is the maximum amount of solute that can be dissolved in a solvent at equilibrium, which produces a saturated solution. Solubility is the property of a substance, known as the solute, that allows it to be dissolved in a solvent. virtually all substances are either partially or completely soluble in a particular solvent. in many cases, a solute can also be a solvent, based on its proportion with a solute.
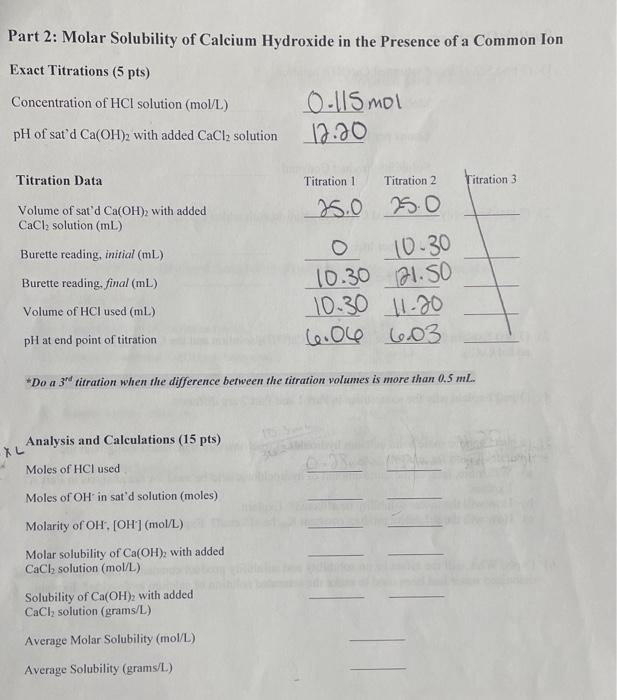
Solved Part 1 Molar Solubility And Solubility Product Of Chegg What is solubility? the maximum amount of solute that can dissolve in a known quantity of solvent at a certain temperature is its solubility. a solution is a homogeneous mixture of one or more solutes in a solvent. sugar cubes added to a cup of tea or coffee are a common example of a solution. The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt. solubility rules. there are a number of patterns in the data obtained from measuring the solubility of different salts. Solubility is defined as the maximum quantity of a substance that can be dissolved in another. it is the maximum amount of solute that can be dissolved in a solvent at equilibrium, which produces a saturated solution. Solubility is the property of a substance, known as the solute, that allows it to be dissolved in a solvent. virtually all substances are either partially or completely soluble in a particular solvent. in many cases, a solute can also be a solvent, based on its proportion with a solute.

Solved Calculate The Molar Solubility S For Each Of The Chegg Solubility is defined as the maximum quantity of a substance that can be dissolved in another. it is the maximum amount of solute that can be dissolved in a solvent at equilibrium, which produces a saturated solution. Solubility is the property of a substance, known as the solute, that allows it to be dissolved in a solvent. virtually all substances are either partially or completely soluble in a particular solvent. in many cases, a solute can also be a solvent, based on its proportion with a solute.

Solution Solubility Products Ksp And Molar Solubility Questions
Comments are closed.