What Is The Lewis Acid Base Model Professor Dave Chegg Explain

Solved Use The Lewis Acid Base Model To Explain The Chegg In this video, we're introducing the lewis model of acids and bases with the help of @professordaveexplains. we'll define lewis acids and lewis bases, compar. With @professordaveexplains, we'll learn about how the lewis model looks at electrons instead of proton transfer, and then we'll cover the concept of an acid base adduct with the.

Solved Lewis Acidlewis Base Chegg With the help of @professordaveexplains, we're delving into the molecular basis of acids and bases through different theoretical models. We'll learn what a base is, summarize different models of acidity and basicity (arrhenius, bronsted lowry), review how to calculate ph, and delve into base strength through the base. The acid and conjugate base as well as the base and conjugate acid are known as conjugate pairs. the acid and base are found on the reactant side of the equation; the conjugate acid and conjugate base are the products. In this video, @professordaveexplains sheds light on the brønsted lowry model to differentiate between strong and weak acids. we'll also explore the location and arrangement of acids on.
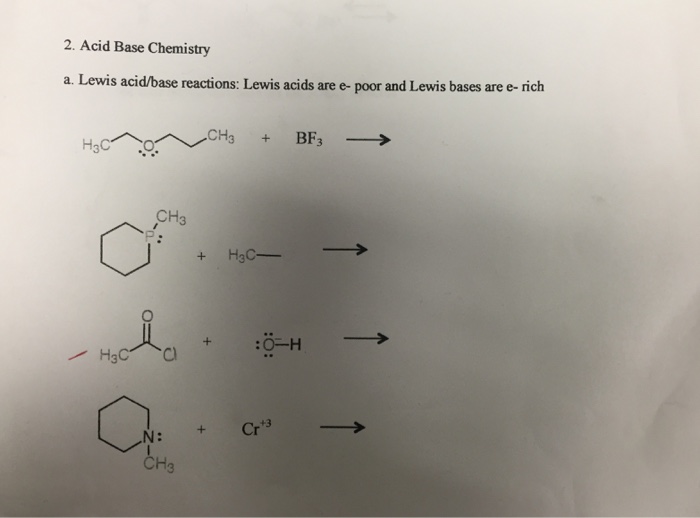
Solved Acid Base Chemistry Lewis Acid Base Reactions Lewis Chegg The acid and conjugate base as well as the base and conjugate acid are known as conjugate pairs. the acid and base are found on the reactant side of the equation; the conjugate acid and conjugate base are the products. In this video, @professordaveexplains sheds light on the brønsted lowry model to differentiate between strong and weak acids. we'll also explore the location and arrangement of acids on. According to lewis: an acid is a substance that accepts a pair of electrons, and in doing so, forms a covalent bond with the entity that supplies the electrons. a base is a substance that donates an unshared pair of electrons to a recipient species with which the electrons can be shared. This page discusses various acid base concepts in chemistry, emphasizing that the choice between them depends on convenience in specific situations, rather than correctness. The species donating the electron pair that compose the bond is a lewis base, the species accepting the electron pair is a lewis acid, and the product of the reaction is a lewis acid base adduct. In this video, @professordaveexplains covers the concept of the ph scale. now that we've learned about acids, bases, and acid base equilibria, we can delve further into these concepts;.

Solved 3 Describe Lewis Acid And Base Specify Following Chegg According to lewis: an acid is a substance that accepts a pair of electrons, and in doing so, forms a covalent bond with the entity that supplies the electrons. a base is a substance that donates an unshared pair of electrons to a recipient species with which the electrons can be shared. This page discusses various acid base concepts in chemistry, emphasizing that the choice between them depends on convenience in specific situations, rather than correctness. The species donating the electron pair that compose the bond is a lewis base, the species accepting the electron pair is a lewis acid, and the product of the reaction is a lewis acid base adduct. In this video, @professordaveexplains covers the concept of the ph scale. now that we've learned about acids, bases, and acid base equilibria, we can delve further into these concepts;.
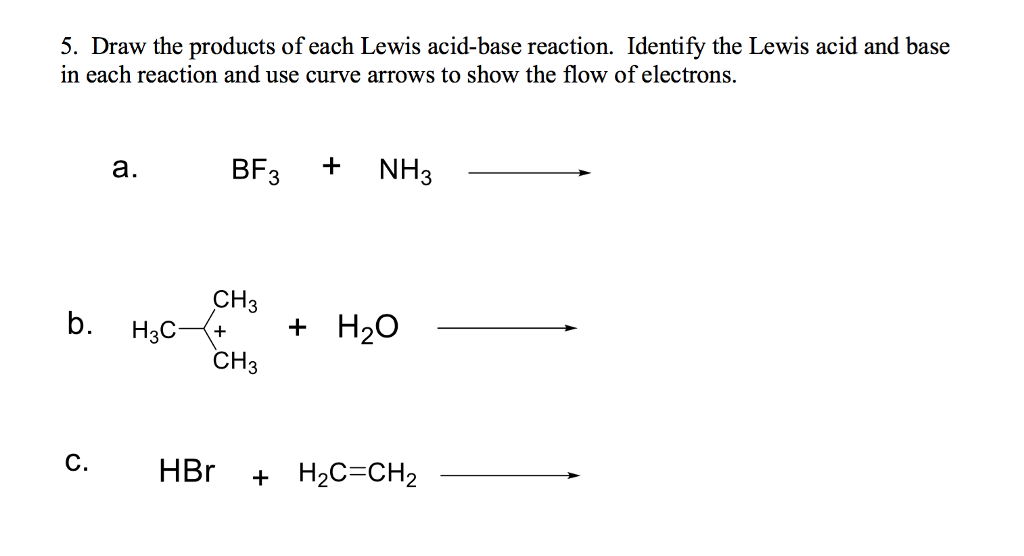
Solved Draw The Products Of Each Lewis Acid Base Reaction Chegg The species donating the electron pair that compose the bond is a lewis base, the species accepting the electron pair is a lewis acid, and the product of the reaction is a lewis acid base adduct. In this video, @professordaveexplains covers the concept of the ph scale. now that we've learned about acids, bases, and acid base equilibria, we can delve further into these concepts;.

A Level Acid Base Theory Lewis Acids And Bases Bronsted Lowry Proton
Comments are closed.