Which Of The Following Compound Will Have The Smallest Pk_a Value

Which Of The Following Compounds Would Have The Smallest Value For Pka Correct answer b (b) formic acid will have the smallest `pk (a)` value and is the storngest acid. Phenyl group is however electron withdrawing but benzoic acid is a weaker acid as compared to formic acid. this is because of r effect of phenyl group, which on the other hand destabilises the carboxylate ion. hence, formic acid (h cooh) is the strongest acid. thus, its pk a value will be lowest.

Solved Which One Of The Following Chemicals Has The Lowest Chegg Smaller the pk (a) value stronger is the acid. as, out of the given acids, formic acid is the strongest acid, its pk (a) will be smallest. Which of the following compounds would have the smallest value of pka p k a? to determine which compound has the smallest pka p k a value (indicating the highest acidity), we need to examine the effect of different substituents on the carboxyl group in the carboxylic acids provided. The presence of electronegative atoms such as fluorine can stabilize the conjugate base through the inductive effect, thereby increasing the acidity of the compound and lowering its pka value. the more fluorine atoms and the closer they are to the carboxylic group, the stronger the inductive effect and the lower the pka value. The presence of electron withdrawing groups near the carboxylic acid group increases the acidity of the compound, thus decreasing the pka value. in this case, the compound with the most electron withdrawing groups near the carboxylic acid group will have the smallest pka value.

Which Of The Following Compound Would Have The Smallest Pka Value Mathr The presence of electronegative atoms such as fluorine can stabilize the conjugate base through the inductive effect, thereby increasing the acidity of the compound and lowering its pka value. the more fluorine atoms and the closer they are to the carboxylic group, the stronger the inductive effect and the lower the pka value. The presence of electron withdrawing groups near the carboxylic acid group increases the acidity of the compound, thus decreasing the pka value. in this case, the compound with the most electron withdrawing groups near the carboxylic acid group will have the smallest pka value. Formic acid is strongest acid among these and hence has smallest pk (a) value . Which of the following compounds would have the smallest value for `pk (a)`? a. `chf (2)ch (2)ch (2)cooh` (2)cooh` d. `ch (3)cf (2)ch (2)cooh`. Order of acidic nature depends on the stability of conjugate base. here the conjugate base stability depends upon the inductive effect of f group. as we know inductive effect is distance dependent effect. in ch3ch2cf 2cooh, two f atoms are close to cooh. (b) formic acid will have the smallest pk (a) value and is the storngest acid.
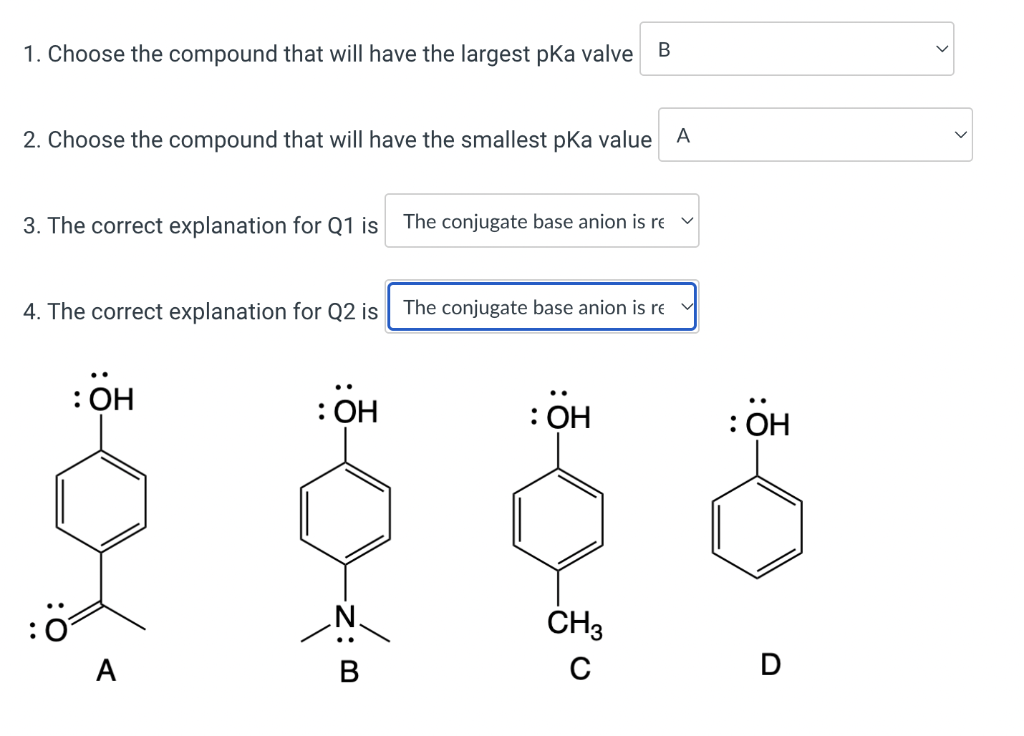
Solved 1 Choose The Compound That Will Have The Largest Pka Chegg Formic acid is strongest acid among these and hence has smallest pk (a) value . Which of the following compounds would have the smallest value for `pk (a)`? a. `chf (2)ch (2)ch (2)cooh` (2)cooh` d. `ch (3)cf (2)ch (2)cooh`. Order of acidic nature depends on the stability of conjugate base. here the conjugate base stability depends upon the inductive effect of f group. as we know inductive effect is distance dependent effect. in ch3ch2cf 2cooh, two f atoms are close to cooh. (b) formic acid will have the smallest pk (a) value and is the storngest acid.
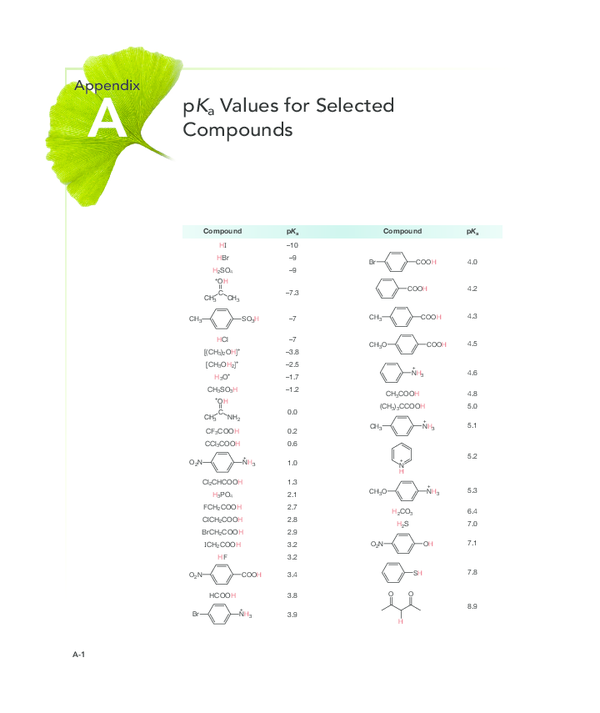
Pdf Pk A Values For Selected Compounds Compound Pk A Compound Pk A Order of acidic nature depends on the stability of conjugate base. here the conjugate base stability depends upon the inductive effect of f group. as we know inductive effect is distance dependent effect. in ch3ch2cf 2cooh, two f atoms are close to cooh. (b) formic acid will have the smallest pk (a) value and is the storngest acid.

Solved 1 Choose The Compound That Will Have The Largest Pka Chegg
Comments are closed.